Bovine spongiform encephalopathy
Bovine spongiform encephalopathy (BSE) is a progressive, fatal disease of the nervous system of bovines that is caused by the accumulation of an abnormal protein called ‘prion’ in nervous tissue. Two forms can be distinguished: classical BSE (caused by C-type BSE agent), which occurs in bovines after ingesting prion contaminated feed, and atypical BSE (caused by H- and L-type agents), which is believed to occur spontaneously in all bovine populations. Classical BSE was first detected in 1986, and the implementation of appropriate control measures resulted in its decline worldwide. To date, the incidence of both forms is negligible and estimated to approach zero cases per million bovines. Classical BSE is considered zoonotic due to its assumed link with the emergence of variant Creutzfeldt-Jakob disease in humans. Classical BSE is a WOAH-listed disease, for which WOAH has established official recognition of sanitary risk status.
Links to Code and Manual
-
Terrestrial code
-
Terrestrial manual
What is BSE?
BSE is a disease of the nervous system of bovines, which has a long incubation period of two to more than ten years. There is currently no treatment or vaccine against it.
It is one of a group of diseases known as transmissible spongiform encephalopathies (TSE), or prion diseases, characterised by the accumulation in nervous tissue of an abnormal infectious protein called a prion. This group notably includes scrapie in sheep and goats, chronic wasting disease (CWD) in cervids, and variant Creutzfeldt-Jakob disease (vCJD) in humans. A neurological disease in domestic and zoo felids has also been linked to BSE.
The hypothesis that BSE prions have been passed to humans, causing vCJD, is strongly supported by epidemiological and clinicopathological studies.
| Classical versus atypical BSE There is a distinction to be made between these two forms: Classical BSE occurs through the consumption of contaminated feed (see section ‘transmission and spread’). Whilst classical BSE was identified as a significant threat in the 90s, its incidence has markedly decreased over the past years, as a result of the successful implementation of effective control measures, and is now estimated to be extremely low (close to 0 cases/year worldwide). Atypical BSE refers to naturally and sporadically occurring forms, which are believed to occur in all bovine populations at a very low rate, and which have only been identified in older bovines when conducting intensive surveillance. In the early 2000s, prions causing atypical BSE were identified as the result of enhanced surveillance for transmissible spongiform encephalopathies. The yearly incidence of atypical BSE is estimated to be negligible. Indeed, whilst to date there is no evidence that atypical BSE is transmissible, recycling of the atypical BSE agent has not been ruled out, and therefore measures to manage exposure risk in the feed chain continue to be recommended as a precautionary measure. |
Classical BSE is a WOAH-listed disease and must be reported to WOAH, as indicated in the WOAH Terrestrial Animal Health Code. Atypical BSE is not a WOAH-listed disease; nevertheless, for the purpose of official recognition and annual reconfirmation of BSE risk status, Members need to provide evidence that any bovines detected with atypical BSE have been completely destroyed or disposed of to ensure that they do not enter the feed or food chain. This is because the potential for recycling of atypical BSE cannot be ruled out and thus should be avoided.
Transmission and spread
The clear understanding of the origin and development of the disease in animals is still subject to scientific research. Nevertheless, it has been proven that certain tissues of infected animals have greater BSE infectivity. According to the WOAH Terrestrial Animal Health Code, these tissues include, according to the bovine’s age: brain, eyes, spinal cord, skull, vertebral column, distal ileum, and any tissue or product that could have been contaminated by or prepared using them.
Scientists believe that bovines are usually infected through the dietary intake of BSE agent-contaminated feed during their first year of life. The risk of contamination occurs if the feed contains products derived from ruminants, such as protein meal derived from ruminants.
The infectious agents are resistant to commercial inactivation procedures such as heat, which means that they may not be destroyed in the rendering process, however their infectivity may be reduced following specific treatment procedures. The incidence of BSE was much greater for dairy than beef bovines, since generally, dairy herds are fed concentrate rations that, before the introduction of stricter controls, contained protein meal derived from ruminants.
Meanwhile, there is no evidence of direct transmission between animals (horizontal transmission) and little data support that BSE is transmitted from mother to offspring (vertical transmission).
Classical BSE was first diagnosed in bovines in the United Kingdom (UK) in 1986, but had probably been present in the country’s bovine population since the 1970s or earlier. It has then been reported in at least 25 countries other than the UK, mainly in Europe, Asia, the Middle East and North America. For more details, see BSE situation in the world.
Nowadays, as a result of the successful implementation of effective control measures, the incidence of classical BSE is extremely low, as well as its global sanitary impact and public health risk.
Public health risk
The likely transmission of classical BSE to humans, assumed to cause vCJD, along with the incapability to predict the size of the vCJD epidemic triggered a public health crisis during the 90s. To date, the number of vCJD clinical cases identified is extremely low.
There are compelling indications that vCJD could be acquired through the consumption of contaminated beef products (as defined below), or contact with medical devices contaminated with classical BSE agents. It is to be noted that dietary exposure to red meat (i.e., deboned skeletal muscle) and milk and milk products is considered safe.
To prevent human and animal infection, and the recycling and amplification of BSE agents, many countries have enforced the systematic removal of tissues with the greatest BSE infectivity, from bovine carcasses. This measure, together with the ban on the use of processed animal proteins in feed (i.e., ruminant-to-ruminant feed ban), have been demonstrated to be strongly efficient in controlling exposure to BSE agents.
The production of human and veterinary pharmaceutical products, and medical devices or cosmetics, should adhere to strict requirements and ideally avoid the use of bovine materials or materials from other animal species in which prion diseases naturally occur.
For more information on vCJD, consult the following references:
• Centers for Disease Control and Prevention
• European Centre for Disease Prevention and Control
Clinical signs
The time between the moment an animal gets infected with a BSE agent and the onset of clinical signs can range from two to more than ten years. Therefore, clinical signs of BSE are found in adult animals, which may demonstrate some of the following clinical signs:
- nervous or aggressive behaviour;
- depression;
- hypersensitivity to sound and touch, twitching, tremors;
- abnormal posture;
- lack of coordination and difficulty in rising from a lying position;
- weight loss, or;
- decreased milk production.
The course of disease is usually subacute to chronic, and affected animals display progressive behavioural or neurological signs. While there have been reports describing specific clinical signs associated with classical versus atypical BSE, it is not possible to discriminate clinically between the two.
There is no effective treatment and affected animals will inevitably die if the disease is left to run its course.
Diagnostic
BSE may be suspected based on clinical signs.
To date, there is no method to confirm the suspicion of BSE in live animals.
As indicated in the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, diagnosis can be reached by prion detection methods such as immunochemical methods including immunohistochemical (IHC) techniques, Western immunoblot methods, and rapid test methods such as lateral flow assays and ELISA. Histopathology is no longer the diagnostic method of choice for investigation of animals, or the screening of healthy populations. Please refer to the BSE Chapter of the Terrestrial Manual for further details.
Prevention and control
In accordance with the Terrestrial Animal Health Code, an effective strategy for preventing the introduction or dealing with occurrences of BSE includes:
- targeted surveillance of bovines that show signs on the clinical spectrum of BSE ;
- transparency in reporting findings of BSE;
- safeguards on importation of relevant commodities;
- removal of tissues with the greatest infectivity (brain, spinal column, etc.) during slaughter and processing of carcasses and from the human food and the animal feed chains;
- prohibition of the inclusion of tissues with the greatest infectivity in animal feeds, thus removing potentially contaminated material from the food chain;
- humane destruction of all suspected animals exposed to feed contaminated with BES agent(s);
- banning the use of ruminant-derived protein meal (ruminant-to-ruminant feed ban, further reinforced by a mammalian-to-ruminant feed ban);
- appropriate disposal of carcasses and all animal products; and
- livestock identification to enable effective surveillance and tracing of suspected livestock.
For more information on targeted BSE surveillance, consult the WOAH guidelines here.
-
Guidelines
BSE_Guidelines_A
.pdf – 658 KB See the document
BSE-risk Status
Classical BSE is a disease for which the WOAH has established official recognition of sanitary risk status in countries in their entirety or in defined zones, through a transparent, science-based and impartial procedure.
WOAH officially recognises two categories of BSE risk status. The current requirements for the recognition and maintenance of these categories can be summarised as follows:
Negligible risk
- Negligible risk of the classical BSE agent being recycled within the bovine population for at least the preceding 8 years;
- Ongoing implementation of a surveillance programme for at least the preceding 8 years;
- The history of occurrence, investigations and management of cases of BSE (classical and atypical) demonstrate either the absence of indigenous case of BSE or the control of the risk of BSE agents being recycled for at least the preceding 8 years;
Controlled risk
- All of the conditions mentioned above are met, but for less than 8 years for one or more of them.
A country or zone that cannot demonstrate that it has met the requirements for ‘controlled’ or ‘negligible’ BSE risk is considered to pose an ‘undetermined’ BSE risk.
‘Atypical BSE’ forms are excluded from the scope of the categorisation, because they are believed to occur spontaneously in all bovine populations at a very low rate.
View the list of WOAH Members with a BSE risk status in the Official Diseases Status section of this page
Last update : 30/05/2023
In accordance with the WOAH procedure for official recognition of disease status, this page provides access to the List of The World Organisation for Animal Health (WOAH) Members officially recognised as having a negligible or controlled bovine spongiform encephalopathy (BSE) risk status by WOAH through the adoption of a resolution by the World Assembly of Delegates (Assembly) of WOAH at the General Session in May every year.
A Member wishing to be officially recognised as having a BSE risk status by WOAH should submit the questionnaire laid out in Chapter 1.6. of the Terrestrial Animal Health Code (Terrestrial Code) and comply with all requirements specified in the Terrestrial Code for BSE. The WOAH Scientific Commission for Animal Diseases (Scientific Commission) is responsible for undertaking, on behalf of the Assembly, the assessment of WOAH Members’ applications for their compliance with WOAH standards. The assessment carried out by the Scientific Commission is based on the recommendations formulated by a relevant ad hoc Group composed of world specialists in disease control.
-
Questionnaire
A_Questionnaire_BSE
.docx – 91 KB See the document
When the Scientific Commission determines that the conditions are not met anymore to demonstrate compliance with the relevant requirements of the Terrestrial Code, a disease status may be suspended. The Scientific Commission may decide to reinstate the suspended status when a Member has submitted an application which fulfils all the requirements requested for the recovery of official disease status described in the relevant Chapters of the Terrestrial Code. The suspensions and recoveries of disease status are announced by the Director General of WOAH in consultation with the Scientific Commission and the list of these is kept up to date until the adoption of a new resolution by the Assembly the following May.
Members with an animal health status officially recognised by WOAH must submit an annual reconfirmation form by the end of November every year.
-
.docx – 38 KB See the document
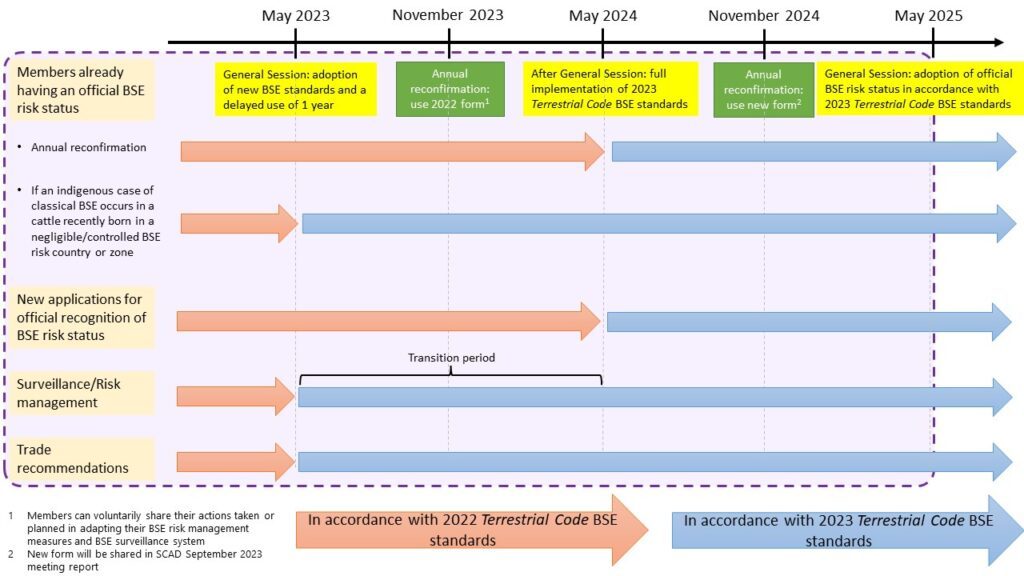
As presented in the figure below, Members applying for, or already having a BSE risk status officially recognised by WOAH should be following the surveillance and risk management practices as per standards adopted in May 2023. For more information on targeted BSE surveillance, consult the WOAH guidelines.
-
Guidelines
BSE_Guidelines_A
.pdf – 658 KB See the document
The figure below summarises the relevant standards and procedure to be followed between May 2023 to May 2025.
Map of BSE official status
List of Members with a BSE risk status
According to Resolution No. 23 (91st General Session, May 2024)
Negligible BSE risk
Members recognised as having a negligible BSE risk in accordance with Chapter 11.4. of the Terrestrial Code :
| Argentina | Germany | Norway |
| Australia | Hungary | Panama |
| Austria | Iceland | Paraguay |
| Belgium | India | Peru |
| Bolivia | Ireland | Poland |
| Brazil | Israel | Portugal (2) |
| Bulgaria | Italy | Romania |
| Canada | Japan | Serbia (3) |
| Chile | Korea (Rep. of) | Singapore |
| Colombia | Latvia | Slovakia |
| Costa Rica | Liechtenstein | Slovenia |
| Croatia | Lithuania | Spain (4) |
| Cyprus | Luxembourg | Sweden |
| Czech Republic | Malta | Switzerland |
| Denmark | Mexico | The Netherlands |
| Estonia | Namibia | United States of America |
| Finland (1) | New Zealand | Uruguay |
| France | Nicaragua |
(2) Including Azores and Madeira.
(3) Excluding Kosovo administered by the United Nations.
(4) Including Balearic Islands and Canary Islands.
Controlled BSE risk
Members recognised as having a controlled BSE risk in accordance with Chapter 11.4. of the Terrestrial Code :
| Chinese Taipei | Greece |
| Ecuador | Russia |
Zone(5) with a negligible BSE risk
Members recognised as having a zone with a negligible BSE risk in accordance with Chapter 11.4. of the Terrestrial Code :
China (People’s Rep. of):
A zone designated by the Delegate of China in a document addressed to the Director General in November 2013, consisting of the People’s Republic of China with the exclusion of Hong Kong and Macau.
United Kingdom (Map)
One zone consisting of Northern Ireland as designated by the Delegate of the United Kingdom in a document addressed to the Director General in September 2016.
One zone consisting of Jersey as designated by the Delegate of the United Kingdom in a document addressed to the Director General in August 2019.
Zone(5) with a controlled BSE risk
Members recognised as having a zone with a controlled BSE risk in accordance with Chapter 11.4. of the Terrestrial Code :
United Kingdom (Map)
One zone consisting of England and Wales as designated by the Delegate of the United Kingdom in documents addressed to the Director General in September and October 2016 and in November 2021;
One zone consisting of Scotland as designated by the Delegate of the United Kingdom in documents addressed to the Director General in September and October 2016 and in December 2018.
(5) For detailed information on the delimitation of the zones of the Members recognised as having a negligible or controlled BSE risk, enquiries should be addressed to the Director General of WOAH.
Years of Members’ official recognition of BSE risk status
With regard to the BSE standards adopted at the 90th General Session in May 2023, the Scientific Commission for Animal Diseases recommended the year of official recognition of Members’ BSE risk status be published to be used as a common reference in estimating the year from which the risk of BSE agents being recycled within the cattle population could be considered as negligible (referred to as the ‘starting date’).
CLICK HERE to find the Table with “Year of official recognition of BSE risk status” and an explanation of how this year should be considered in estimating the ‘starting date’.




