Early-warning systems: modeling the spread of vector-borne diseases

Annamaria Conte is Head of the Statistics and Geographic Information System Unit at institute Zooprofilattico Sperimentale (IZS) dell’Abruzzo e del Molise “G. Caporale”-Teramo.
Paolo Calistri is Head of the Epidemiology and Public Health Department at Institute IZS-Teramo.
How do vector-borne diseases such as Rift Valley fever spread?
Paolo Calistri: Rift Valley fever is a vector-borne disease transmitted by infected mosquito bites. The disease affects domesticated animals such as buffalo, camels, cattle, goats, and sheep, while wild animals can act as reservoirs of the virus in some African areas. The disease can also affect humans. It can spread geographically with the movement of infected animals, but this route of dissemination can be controlled through international trade regulations. Another way is through infected vectors: in this case, several species of mosquitoes have been identified as capable of transmitting the virus. No need to say that there are no control measures to block mosquitoes from flying across national borders! Through wind or other passive mechanisms (inside airplanes, sea cargos, etc.), they can travel long distances.
The PROVNA project (“Defining Ecoregions and Prototyping on Earth Observation (EO)-based Vector-borne Disease Surveillance System for North Africa”), launched under WOAH’s initiative, aims to help countries in North Africa target their surveillance on Rift Valley fever, by tapping into remote sensing and earth observation data. By helping North African countries build an early-warning system and keep the spread of Rift Valley fever under control, we are helping everyone, including in other neighbouring regions such as Mediterranean Europe and Middle East.
Why are environmental and climate data helpful to predict the movement of mosquito populations?
Annamaria Conte: We are aiming to identify ecoregions in North Africa, with similar characteristics in terms of temperature, environment, vegetation and moisture of the soil, notably. We use earth observation data from NASA and high-resolution data from the European programme Copernicus, combined with data on animal population and occurrence of Rift Valley fever, extracted from the World Animal Health Information System (WAHIS) and FAO Empres-I . If infected mosquitoes appear in one of these areas, we can expect similar areas to be able to house the virus as well.
We combine spatio-temporal data to build a prototype which should be able to predict the locations of areas at risk, and when the risk might occur. We take into account past conditions of temperature, rainfall and vegetation: we look at the past to forecast the future.
What kinds of expertise are required to design such a model?
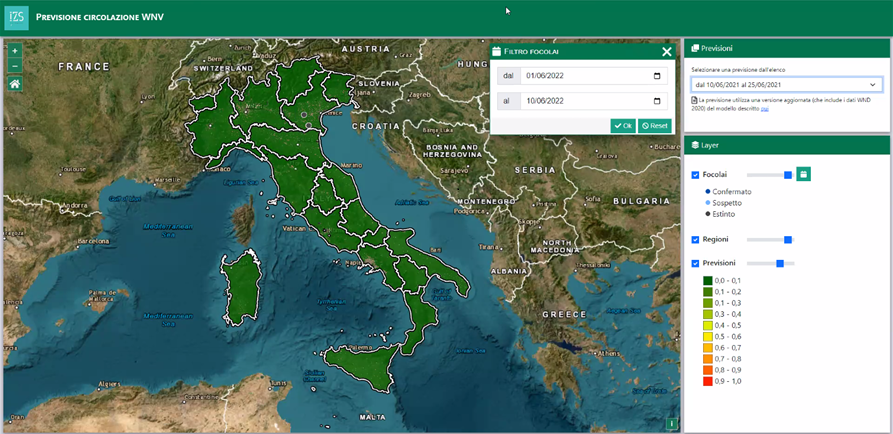
A. C. The group I lead is composed of mathematicians, statisticians and geographers. We collaborate with Paolo’s team of veterinarians and epidemiologists, so it’s a perfect transdisciplinary environment. Together, we identify the most relevant indicators to each specific disease from earth observation data and we apply machine learning and artificial intelligence techniques to map out the areas at risk of infection. Our first model concerned the spread of West Nile fever in Italy: we were able to predict 15 days in advance where the climatic and environmental conditions favorable to the virus spread were most likely to occur. With PROVNA, we are developing a similar model for Rift Valley fever in North Africa.
How is this information going to be used to contain vector-borne diseases?
P. C. We cannot stop the mosquitoes. However, if we know in advance when and where mosquitos are most likely to spread and potentially infect livestock, we can plan vaccination campaigns and emergency measures at the right time and place. The model provides us with an efficient early-warning system, that can be associated with a contingency plan. For example, hospitals can know when and where the mosquito season will start this year, and get ready.

Can the model be adapted to other diseases?
A. C. We can apply a similar methodology, as we did for West Nile fever to the Rift Valley fever, and develop a similar model using different datasets. But we need to know which variables play a role in the disease spread, since each vector-borne disease has specific vectors influenced by certain factors. We will look at different environmental and climate characteristics to spot different species of mosquitoes, or other vectors, such as ticks. This is why it is so important to work in a transdisciplinary way, with epidemiologists, entomologists, as well as statisticians.
How do you plan to work with national veterinary authorities?
P. C. If all goes well, the model will be available in the summer of 2023. With WOAH’s support, we got in touch with national Veterinary Services in North African countries to organise training sessions, and to discuss how the model can be improved with their own field data, for example quantitative entomological data. The more information we feed into the model, the more accurate it will be. Once they get ahold of the model, they can use it to better focus surveillance of Rift Valley fever, and plan the best use of their resources in case of an emergency.
