Terrestrial Animal Health Code
Terrestrial Animal Health Code |
Infection with avian influenza viruses
General provisions
For the purposes of the Terrestrial Code, avian influenza is defined as an infection of poultry caused by any influenza A virus of the H5 or H7 subtypes or by any influenza A virus with an intravenous pathogenicity index (IVPI) greater than 1.2 (or as an alternative at least 75% mortality) as described below. These viruses are divided into high pathogenicity avian influenza viruses and low pathogenicity avian influenza viruses:
high pathogenicity avian influenza viruses have an IVPI in six-week-old chickens greater than 1.2 or, as an alternative, cause at least 75% mortality in four-to eight-week-old chickens infected intravenously. H5 and H7 viruses which do not have an IVPI of greater than 1.2 or cause less than 75% mortality in an intravenous lethality test should be sequenced to determine whether multiple basic amino acids are present at the cleavage site of the haemagglutinin molecule (HA0); if the amino acid motif is similar to that observed for other high pathogenicity avian influenza isolates, the isolate being tested should be considered as high pathogenicity avian influenza virus;
low pathogenicity avian influenza viruses are all influenza A viruses of H5 and H7 subtypes that are not high pathogenicity avian influenza viruses.
The following defines the occurrence of infection with an avian influenza virus: the virus has been isolated and identified as such or specific viral ribonucleic acid has been detected in poultry or a product derived from poultry.
Poultry is defined as ‘all domesticated birds, including backyard poultry, used for the production of meat or eggs for consumption, for the production of other commercial products, for restocking supplies of game, or for breeding these categories of birds, as well as fighting cocks used for any purpose’.
Birds that are kept in captivity for any reason other than those reasons referred to in the preceding paragraph, including those that are kept for shows, races, exhibitions, competitions or for breeding or selling these categories of birds as well as pet birds, are not considered to be poultry.
For the purposes of the Terrestrial Code, the incubation period for avian influenza shall be 21 days.
This chapter deals not only with the occurrence of clinical signs caused by avian influenza, but also with the presence of infection with avian influenza viruses in the absence of clinical signs.
Antibodies against H5 or H7 subtype, which have been detected in poultry and are not a consequence of vaccination, should be immediately investigated. In the case of isolated serological positive results, infection with avian influenza viruses may be ruled out on the basis of a thorough epidemiological and laboratory investigation that does not demonstrate further evidence of such an infection.
For the purposes of the Terrestrial Code, ‘avian influenza free establishment’ means an establishment in which the poultry have shown no evidence of infection with avian influenza viruses, based on surveillance in accordance with Articles 10.4.27. to 10.4.33.
Infection with influenza A viruses of high pathogenicity in birds other than poultry, including wild birds, should be notified in accordance with Article 1.1.3. However, a Member Country should not impose bans on the trade in poultry and poultrycommodities in response to such a notification, or other information on the presence of any influenza A virus in birds other than poultry, including wild birds.
Standards for diagnostic tests, including pathogenicity testing, are described in the Terrestrial Manual. Any vaccine used should comply with the standards described in the Terrestrial Manual.
Determination of the avian influenza status of a country, zone or compartment
The avian influenza status of a country, a zone or a compartment can be determined on the basis of the following criteria:
avian influenza is notifiable in the whole country, an ongoing avian influenza awareness programme is in place, and all notified suspect occurrences of avian influenza are subjected to field and, where applicable, laboratory investigations;
appropriate surveillance is in place to demonstrate the presence of infection in the absence of clinical signs in poultry, and the risk posed by birds other than poultry; this may be achieved through an avian influenza surveillance programme in accordance with Articles 10.4.27. to 10.4.33.;
consideration of all epidemiological factors for avian influenza occurrence and their historical perspective.
Country, zone or compartment free from avian influenza
A country, zone or compartment may be considered free from avian influenza when it has been shown that infection with avian influenza viruses in poultry has not been present in the country, zone or compartment for the past 12 months, based on surveillance in accordance with Articles 10.4.27. to 10.4.33.
If infection has occurred in poultry in a previously free country, zone or compartment, avian influenza free status can be regained:
In the case of infections with high pathogenicity avian influenza viruses, three months after a stamping-out policy (including disinfection of all affected establishments) is applied, providing that surveillance in accordance with Articles 10.4.27. to 10.4.33. has been carried out during that three-month period.
In the case of infections with low pathogenicity avian influenza viruses, poultry may be kept for slaughter for human consumption subject to conditions specified in Article 10.4.19. or a stamping-out policy may be applied; in either case, three months after the disinfection of all affected establishments, providing that surveillance in accordance with Articles 10.4.27. to 10.4.33. has been carried out during that three-month period.
Country, zone or compartment free from infection with high pathogenicity avian influenza viruses in poultry
A country, zone or compartment may be considered free from infection with high pathogenicity avian influenza viruses in poultry when:
it has been shown that infection with high pathogenicity avian influenza viruses in poultry has not been present in the country, zone or compartment for the past 12 months, although its status with respect to low pathogenicity avian influenza viruses may be unknown; or
when, based on surveillance in accordance with Articles 10.4.27. to 10.4.33., it does not meet the criteria for freedom from avian influenza but any virus detected has not been identified as high pathogenicity avian influenza virus.
The surveillance may need to be adapted to parts of the country or existing zones or compartments depending on historical or geographical factors, industry structure, population data, or proximity to recent outbreaks.
If infection has occurred in poultry in a previously free country, zone or compartment, the free status can be regained three months after a stamping-out policy (including disinfection of all affected establishments) is applied, providing that surveillance in accordance with Articles 10.4.27. to 10.4.33. has been carried out during that three-month period.
Recommendations for importation from a country, zone or compartment free from avian influenza
For live poultry (other than day-old poultry)
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the poultry showed no clinical sign of avian influenza on the day of shipment;
the poultry were kept in an avian influenza free country, zone or compartment since they were hatched or for at least the past 21 days;
the poultry are transported in new or appropriately sanitized containers.
If the poultry have been vaccinated against avian influenza, the nature of the vaccine used and the date of vaccination should be attached to the certificate.
Recommendations for the importation of live birds other than poultry
Regardless of the avian influenza status of the country of origin, Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
on the day of shipment, the birds showed no clinical sign of infection with a virus which would be considered avian influenza in poultry;
the birds were kept in isolation approved by the Veterinary Services since they were hatched or for at least 21 days prior to shipment and showed no clinical sign of infection with a virus which would be considered avian influenza in poultry during the isolation period;
a statistically valid sample of the birds, selected in accordance with Article 10.4.29., was subjected to a diagnostic test within 14 days prior to shipment to demonstrate freedom from infection with a virus which would be considered avian influenza in poultry;
the birds are transported in new or appropriately sanitized containers.
If the birds have been vaccinated against avian influenza, the nature of the vaccine used and the date of vaccination should be attached to the certificate.
Recommendations for importation from a country, zone or compartment free from avian influenza
For day-old live poultry
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the poultry were kept in an avian influenza free country, zone or compartment since they were hatched;
the poultry were derived from parent flocks which had been kept in an avian influenza free country, zone or compartment for at least 21 days prior to and at the time of the collection of the eggs;
the poultry are transported in new or appropriately sanitized containers.
If the poultry or the parent flocks have been vaccinated against avian influenza, the nature of the vaccine used and the date of vaccination should be attached to the certificate.
Recommendations for importation from a country, zone or compartment free from infection with high pathogenicity avian influenza viruses in poultry
For day-old live poultry
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the poultry were kept in a country, zone or compartment free from infection with high pathogenicity avian influenza viruses in poultry since they were hatched;
the poultry were derived from parent flocks which had been kept in an avian influenza free establishment for at least 21 days prior to and at the time of the collection of the eggs;
the poultry are transported in new or appropriately sanitized containers.
If the poultry or the parent flocks have been vaccinated against avian influenza, the nature of the vaccine used and the date of vaccination should be attached to the certificate.
Recommendations for the importation of day-old live birds other than poultry
Regardless of the avian influenza status of the country of origin, Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
on the day of shipment, the birds showed no clinical sign of infection with a virus which would be considered avian influenza in poultry;
the birds were hatched and kept in isolation approved by the Veterinary Services;
the parent flock birds were subjected to a diagnostic test at the time of the collection of the eggs to demonstrate freedom from infection with a virus which would be considered avian influenza in poultry;
the birds are transported in new or appropriately sanitized containers.
If the birds or parent flocks have been vaccinated against avian influenza, the nature of the vaccine used and the date of vaccination should be attached to the certificate.
Recommendations for importation from a country, zone or compartment free from avian influenza
For hatching eggs of poultry
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the eggs came from an avian influenza free country, zone or compartment;
the eggs were derived from parent flocks which had been kept in an avian influenza free country, zone or compartment for at least 21 days prior to and at the time of the collection of the eggs;
the eggs are transported in new or appropriately sanitized packaging materials.
If the parent flocks have been vaccinated against avian influenza, the nature of the vaccine used and the date of vaccination should be attached to the certificate.
Recommendations for importation from a country, zone or compartment free from infection with high pathogenicity avian influenza viruses in poultry
For hatching eggs of poultry
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the eggs came from a country, zone or compartment free from infection with high pathogenicity avian influenza viruses in poultry;
the eggs were derived from parent flocks which had been kept in an avian influenza free establishment for at least 21 days prior to and at the time of the collection of the eggs;
the eggs have had their surfaces sanitized (in accordance with Chapter 6.5.);
the eggs are transported in new or appropriately sanitized packaging materials.
If the parent flocks have been vaccinated against avian influenza, the nature of the vaccine used and the date of vaccination should be attached to the certificate.
Recommendations for the importation of hatching eggs from birds other than poultry
Regardless of the avian influenza status of the country of origin, Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the parent flock birds were subjected to a diagnostic test seven days prior to and at the time of the collection of the eggs to demonstrate freedom from infection with a virus which would be considered avian influenza in poultry;
the eggs have had their surfaces sanitized (in accordance with Chapter 6.5.);
the eggs are transported in new or appropriately sanitized packaging materials.
If the parent flocks have been vaccinated against avian influenza, the nature of the vaccine used and the date of vaccination should be attached to the certificate.
Recommendations for importation from a country, zone or compartment free from avian influenza
For eggs for human consumption
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the eggs were produced and packed in an avian influenza free country, zone or compartment;
the eggs are transported in new or appropriately sanitized packaging materials.
Recommendations for importation from a country, zone or compartment free from infection with high pathogenicity avian influenza viruses in poultry
For eggs for human consumption
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the eggs were produced and packed in a country, zone or compartment free from infection with high pathogenicity avian influenza viruses in poultry;
the eggs have had their surfaces sanitized (in accordance with Chapter 6.5.);
the eggs are transported in new or appropriately sanitized packaging materials.
Recommendations for importation of egg products of poultry
Regardless of the avian influenza status of the country of origin, Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the commodity is derived from eggs which meet the requirements of Articles 10.4.13. or 10.4.14.; or
the commodity has been processed to ensure the destruction of avian influenza virus in accordance with Article 10.4.25.;
AND
the necessary precautions were taken to avoid contact of the commodity with any source of avian influenza virus.
Recommendations for importation from a country, zone or compartment free from avian influenza
For poultry semen
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that the donor poultry:
showed no clinical sign of avian influenza on the day of semen collection;
were kept in an avian influenza free country, zone or compartment for at least 21 days prior to and at the time of semen collection.
Recommendations for the importation from a country, zone or compartment free from infection with high pathogenicity avian influenza viruses in poultry
For poultry semen
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that the donor poultry:
showed no clinical sign of infection with high pathogenicity avian influenza viruses in poultry on the day of semen collection;
were kept in a country, zone or compartment free from infection with high pathogenicity avian influenza viruses in poultry for at least 21 days prior to and at the time of semen collection.
Recommendations for the importation of semen of birds other than poultry
Regardless of the avian influenza status of the country of origin, Veterinary Authorities should require the presentation of an international veterinary certificate attesting that the donor birds:
were kept in isolation approved by the Veterinary Services for at least 21 days prior to semen collection;
showed no clinical sign of infection with a virus which would be considered avian influenza in poultry during the isolation period;
were tested within 14 days prior to semen collection and shown to be free from infection with a virus which would be considered avian influenza in poultry.
Recommendations for importation from a country, zone or compartment free from avian influenza or free from infection with high pathogenicity avian influenza viruses in poultry
For fresh meat of poultry
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that the entire consignment of fresh meat comes from poultry:
which have been kept in a country, zone or compartment free from infection with high pathogenicity avian influenza viruses in poultry since they were hatched or for at least the past 21 days;
which have been slaughtered in an approved abattoir in a country, zone or compartment free from infection with high pathogenicity avian influenza viruses in poultry and have been subjected to ante- and post-mortem inspections in accordance with Chapter 6.3. and have been found free of any signs suggestive of avian influenza.
Recommendations for the importation of meat products of poultry
Regardless of the avian influenza status of the country of origin, Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the commodity is derived from fresh meat which meets the requirements of Article 10.4.19.; or
the commodity has been processed to ensure the destruction of avian influenza virus in accordance with Article 10.4.26.;
AND
the necessary precautions were taken to avoid contact of the commodity with any source of avian influenza virus.
Recommendations for the importation of products of poultry origin, other than feather meal and poultry meal, intended for use in animal feeding, or for agricultural or industrial use
Regardless of the avian influenza status of the country of origin, Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
these commodities were processed in an avian influenza free country, zone or compartment from poultry which were kept in an avian influenza free country, zone or compartment from the time they were hatched until the time of slaughter or for at least the 21 days preceding slaughter; or
these commodities have been processed to ensure the destruction of avian influenza virus using:
moist heat treatment for 30 minutes at 56°C; or
any equivalent treatment which has been demonstrated to inactivate avian influenza virus;
AND
the necessary precautions were taken to avoid contact of the commodity with any source of avian influenza virus.
Recommendations for the importation of feathers and down of poultry
Regardless of the avian influenza status of the country of origin, Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
these commodities originated from poultry as described in Article 10.4.19. and were processed in an avian influenza free country, zone or compartment; or
these commodities have been processed to ensure the destruction of avian influenza virus using one of the following:
washed and steam-dried at 100ºC for 30 minutes;
fumigation with formalin (10% formaldehyde) for 8 hours;
irradiation with a dose of 20 kilogray;
any equivalent treatment which has been demonstrated to inactivate avian influenza virus;
AND
the necessary precautions were taken to avoid contact of the commodity with any source of avian influenza virus.
Recommendations for the importation of feathers and down of birds other than poultry
Regardless of the avian influenza status of the country of origin, Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
these commodities have been processed to ensure the destruction of any virus which would be considered avian influenza in poultry using one of the following:
washed and steam-dried at 100ºC for 30 minutes;
fumigation with formalin (10% formaldehyde) for 8 hours;
irradiation with a dose of 20 kilogray;
any equivalent treatment which has been demonstrated to inactivate avian influenza virus;
the necessary precautions were taken to avoid contact of the commodity with any source of viruses which would be considered avian influenza in poultry.
Recommendations for the importation of feather meal and poultry meal
Regardless of the avian influenza status of the
country of origin, Veterinary Authorities should
require the presentation of an international veterinary certificate attesting
that:
these commodities were
processed in an avian influenza free country, zone or compartment from poultry which were
kept in an avian influenza free country, zone or compartment from
the time they were hatched until the time of slaughter or
for at least the 21 days preceding slaughter; or
these commodities have
been processed either:
with moist heat at a minimum
temperature of 118ºC for minimum of 40 minutes; or
with a continuous hydrolysing process under
at least 3.79 bar of pressure with steam at a minimum temperature
of 122ºC for a minimum of 15 minutes; or
with an alternative rendering process that ensures that the internal temperature throughout the product reaches at least 74ºC;
AND
the necessary precautions were taken to avoid contact of the commodity with any source of avian influenza viruses.
Procedures for the inactivation of avian influenza viruses in eggs and egg products
The following times for industry standard temperatures are suitable for the inactivation of avian influenza viruses present in eggs and egg products:
| Core temperature (°C) | Time | |
|---|---|---|
| Whole egg | 60 | 188 seconds |
| Whole egg blends | 60 | 188 seconds |
| Whole egg blends | 61.1 | 94 seconds |
| Liquid egg white | 55.6 | 870 seconds |
| Liquid egg white | 56.7 | 232 seconds |
| Plain or pure egg yolk | 60 | 288 seconds |
| 10% salted yolk | 62.2 | 138 seconds |
| Dried egg white | 67 | 20 hours |
| Dried egg white | 54.4 | 50.4 hours |
| Dried egg white | 51.7 | 73.2 hours |
The listed temperatures are indicative of a range that achieves a 7-log kill of avian influenza virus. These are listed as examples in a variety of egg products, but when scientifically documented, variances from these times and temperatures and for additional egg products may also be suitable when they achieve equivalent inactivation of the virus.
Procedures for the inactivation of avian influenza viruses in meat
The following times for industry standard temperatures are suitable for the inactivation of avian influenza viruses present in meat.
| Core temperature (°C) | Time | |
|---|---|---|
| Poultry meat | 60.0 | 507 seconds |
| 65.0 | 42 seconds | |
| 70.0 | 3.5 seconds | |
| 73.9 | 0.51 second |
The listed temperatures are indicative of a range that achieves a 7-log kill. Where scientifically documented, variances from these times and temperatures may also be suitable when they achieve the inactivation of the virus.
Introduction to surveillance
Articles 10.4.27. to 10.4.33. define the principles and provide a guide on the surveillance for avian influenza complementary to Chapter 1.4., applicable to Member Countries seeking to determine their avian influenza status. This may be for the entire country, zone or compartment. Guidance for Member Countries seeking free status following an outbreak and for the maintenance of avian influenza status is also provided.
The presence of influenza A viruses in wild birds creates a particular problem. In essence, no Member Country can declare itself free from influenza A in wild birds. However, the definition of avian influenza in this chapter refers to the infection in poultry only, and Articles 10.4.27. to 10.4.33. were developed under this definition.
The impact and epidemiology of avian influenza differ widely in different regions of the world and therefore it is impossible to provide specific recommendations for all situations. Surveillance strategies employed for demonstrating freedom from avian influenza at an acceptable level of confidence should be adapted to the local situation. Variables such as the frequency of contacts of poultry with wild birds, different biosecurity levels and production systems and the commingling of different susceptible species including domestic waterfowl require specific surveillance strategies to address each specific situation. It is incumbent upon the Member Country to provide scientific data that explains the epidemiology of avian influenza in the region concerned and also demonstrates how all the risk factors are managed. There is therefore considerable latitude available to Member Countries to provide a well-reasoned argument to prove that absence of infection with avian influenza viruses is assured at an acceptable level of confidence.
Surveillance for avian influenza should be in the form of a continuing programme designed to establish that the country, zone or compartment, for which application is made, is free from infection with avian influenza viruses.
General conditions and methods for surveillance
A surveillance system in accordance with Chapter 1.4. should be under the responsibility of the Veterinary Authority. In particular:
a formal and ongoing system for detecting and investigating outbreaks of disease or infection with avian influenza viruses should be in place;
a procedure should be in place for the rapid collection and transport of samples from suspect cases of avian influenza to a laboratory for avian influenza diagnosis;
a system for recording, managing and analysing diagnostic and surveillance data should be in place.
The avian influenza surveillance programme should:
include an early warning system
throughout the production, marketing and processing chain for reporting suspicious
cases. Farmers and workers, who have day-to-day contact with poultry,
as well as diagnosticians, should report promptly any suspicion
of avian influenza to the Veterinary Authority.
They should be supported directly or indirectly (e.g. through private veterinarians or veterinary paraprofessionals)
by government information programmes and the Veterinary Authority.
All suspected cases of avian influenza should be investigated immediately.
As suspicion cannot always be resolved by epidemiological and clinical investigation
alone, samples should be taken and submitted to a laboratory for
appropriate tests. This requires that sampling kits and other equipment
are available for those responsible for surveillance.
Personnel responsible for surveillance should
be able to call for assistance from a team with expertise in avian
influenza diagnosis and control. In cases where potential public
health implications are suspected, notification to the appropriate
public health authorities is essential;
implement, when relevant, regular and frequent
clinical inspection and serological and virological testing of high-risk
groups of animals, such as those adjacent to an avian influenza
infected country or zone, places where
birds and poultry of different
origins are mixed, such as live bird markets, poultry in
close proximity to waterfowl or other potential sources of influenza
A viruses.
An effective surveillance system will periodically identify suspicious cases that require follow-up and investigation to confirm or exclude that the cause of the condition is influenza A viruses. The rate at which such suspicious cases are likely to occur will differ between epidemiological situations and cannot therefore be predicted reliably. Documentation for freedom from infection with avian influenza viruses should, in consequence, provide details of the occurrence of suspicious cases and how they were investigated and dealt with. This should include the results of laboratory testing and the control measures to which the animals concerned were subjected during the investigation (quarantine, movement stand-still orders, etc.).
Surveillance strategies
Introduction
The target population for surveillance aimed
at identification of disease and infection should
cover all the susceptible poultry species
within the country, zone or compartment.
Active and passive surveillance for
avian influenza should be ongoing, with the frequency of active surveillance being
appropriate to the epidemiological situation in the country. Surveillance should
be composed of random and targeted approaches using molecular, virological,
serological and clinical methods.
The strategy employed may be based on randomised
sampling requiring surveillance consistent
with demonstrating the absence of infection with
avian influenza viruses at an acceptable level of confidence. Random surveillance is
conducted using serological tests. Positive serological results
should be followed up with molecular or virological methods.
Targeted surveillance (e.g.
based on the increased likelihood of infection in particular
localities or species) may be an appropriate strategy. Virological
and serological methods should be used concurrently to define the
avian influenza status of high risk populations.
A Member Country should justify the surveillance strategy
chosen as adequate to detect the presence of infection with
avian influenza viruses in accordance with Chapter 1.4. and
the prevailing epidemiological situation, including cases of
high pathogenicity influenza A detected in any birds. It may, for
example, be appropriate to target clinical surveillance at
particular species likely to exhibit clear clinical signs (e.g.
chickens). Similarly, virological and serological testing could
be targeted to species that may not show clinical signs (e.g. ducks).
If a Member Country wishes to declare freedom
from infection with
avian influenza viruses in a specific zone or compartment,
the design of the survey and the basis for the sampling process
would need to be aimed at the population within the zone or compartment.
For random surveys, the design of the sampling strategy should incorporate epidemiologically appropriate design prevalence. The sample size selected for testing should be large enough to detect infection if it were to occur at a predetermined minimum rate. The sample size and expected disease prevalence determine the level of confidence in the results of the survey. The Member Country should justify the choice of design prevalence and confidence level based on the objectives of surveillance and the epidemiological situation, in accordance with Chapter 1.4. Selection of the design prevalence in particular should be clearly based on the prevailing or historical epidemiological situation.
Irrespective of the survey approach selected,
the sensitivity and specificity of the diagnostic tests employed
are key factors in the design, sample size determination and interpretation
of the results obtained. Ideally, the sensitivity and specificity
of the tests used should be validated for the vaccination and infection history
and the different species in the target population.
Irrespective of the testing system employed, surveillance system
design should anticipate the occurrence of false positive reactions.
If the characteristics of the testing system are known, the rate
at which these false positives are likely to occur can be calculated
in advance. There should be an effective procedure for following
up positives to ultimately determine with a high level of confidence,
whether they are indicative of infection or not.
This should involve both supplementary tests and follow-up investigation
to collect diagnostic material from the original sampling unit as
well as flocks which
may be epidemiologically linked to it.
The principles involved in surveillance for
disease and infection are
technically well defined. The design of surveillance programmes
to prove the absence of infection with,
or circulation of, avian influenza viruses should be carefully followed
to avoid producing results that are either insufficiently reliable,
or excessively costly and logistically complicated. The design of
any surveillance programme,
therefore, requires inputs from professionals competent and experienced
in this field.
Clinical surveillance
Clinical surveillance aims
at the detection of clinical signs of avian influenza at the flock level.
Whereas significant emphasis is placed on the diagnostic value of
mass serological screening, surveillance based
on clinical inspection should not be underrated. Monitoring of production
parameters, such as increased mortality, reduced feed and water
consumption, presence of clinical signs of a respiratory disease
or a drop in egg production, is important for the early detection
of infection with
avian influenza viruses. In some cases, the only indication of infection with
low pathogenicity avian influenza virus may be a drop in feed consumption
or egg production.
Clinical surveillance and laboratory testing
should always be applied in series to clarify the status of avian
influenza suspects detected by either of these complementary diagnostic
approaches. Laboratory testing
may confirm clinical suspicion, while clinical surveillance may
contribute to confirmation of positive serology. Any sampling unit within
which suspicious animals are detected should have restrictions imposed
upon it until avian influenza infection is ruled
out.
Identification of suspect flocks is vital to the identification of sources of avian influenza viruses and to enable the molecular, antigenic and other biological characteristics of the virus to be determined. It is essential that avian influenza virus isolates are sent regularly to the regional Reference Laboratory for genetic and antigenic characterisation.
Virological surveillance
Virological surveillance should
be conducted:
to monitor at risk populations;
to confirm clinically suspect cases;
to follow up positive serological results;
to test ‘normal’ daily mortality, to ensure early detection of infection in the face of vaccination or in establishments epidemiologically linked to an outbreak.
Serological surveillance
Serological surveillance aims
at the detection of antibodies against avian influenza virus. Positive
avian influenza viruses antibody test results can have four possible
causes:
natural infection with
avian influenza viruses;
vaccination against
avian influenza;
maternal antibodies derived from a vaccinated
or infected parent flock are usually
found in the yolk and can persist in progeny for up to four weeks;
lack of specificity of the test.
It may be possible to use serum collected for
other survey purposes for avian influenza surveillance.
However, the principles of survey design described in these recommendations
and the requirement for a statistically valid survey for the presence
of avian influenza viruses should not be compromised.
The discovery of clusters of seropositive flocks may
reflect any of a series of events, including but not limited to
the demographics of the population sampled, vaccinal exposure or infection.
As clustering may signal infection, the investigation
of all instances should be incorporated in the survey design. Clustering
of positive flocks is always epidemiologically
significant and therefore should be investigated.
If vaccination cannot
be excluded as the cause of positive serological reactions, diagnostic
methods to differentiate antibodies due to infection or vaccination should
be employed.
The results of random or targeted serological
surveys are important in providing reliable evidence that no infection with
avian influenza viruses is present in a country, zone or compartment.
It is therefore essential that the survey be thoroughly documented.
Virological and serological surveillance
in vaccinated populations
The surveillance strategy
is dependent on the type of vaccine used. The protection against
influenza A virus is haemagglutinin subtype specific. Therefore,
two broad vaccination strategies
exist: 1) inactivated whole viruses, and 2) haemagglutinin expression-based
vaccines.
In the case of vaccinated populations, the surveillance strategy
should be based on virological or serological methods and clinical surveillance.
It may be appropriate to use sentinel birds for this purpose. These
birds should be unvaccinated, virus antibody free birds and clearly
and permanently identified. Sentinel birds should be used only if
no appropriate laboratory procedures
are available. The interpretation of serological results in the
presence of vaccination is
described in Article 10.4.33.
Documentation of freedom from avian influenza
or freedom from infection with high pathogenicity avian influenza viruses
in poultry
Additional surveillance
requirements for Member Countries declaring freedom of the country,
zone or compartment from avian influenza or from infection with
high pathogenicity avian influenza viruses in poultry
In addition to the general conditions described in above mentioned articles, a Member Country declaring freedom of the entire country, or a zone or a compartment from avian influenza or from infection with high pathogenicity avian influenza viruses in poultry should provide evidence for the existence of an effective surveillance programme.
The strategy and design of the surveillance programme depend on the prevailing epidemiological circumstances and should be planned and implemented in accordance with general conditions and methods described in this chapter, to demonstrate absence of infection with avian influenza viruses or with high pathogenicity avian influenza viruses, during the preceding 12 months in susceptible poultry populations (vaccinated and non-vaccinated). This requires the support of a laboratory able to undertake identification of infection with avian influenza viruses through virus detection and antibody tests. This surveillance may be targeted to poultry population at specific risks linked to the types of production, possible direct or indirect contact with wild birds, multi-age flocks, local trade patterns including live bird markets, use of possibly contaminated surface water, and the presence of more than one species on the holding and poor biosecurity measures in place.
Additional requirements for countries, zones or compartments that practise vaccination
Vaccination to prevent the transmission of high pathogenicity avian influenza virus may be part of a disease control programme. The level of flock immunity required to prevent transmission depends on the flock size, composition (e.g. species) and density of the susceptible poultry population. It is therefore impossible to be prescriptive. Based on the epidemiology of avian influenza in the country, zone or compartment, it may be that a decision is reached to vaccinate only certain species or other poultry subpopulations.
In all vaccinated flocks there is a need to perform virological and serological tests to ensure the absence of virus circulation. The use of sentinel poultry may provide further confidence of the absence of virus circulation. The tests have to be repeated at least every six months or at shorter intervals in accordance with the risk in the country, zone or compartment.
Evidence to show the effectiveness of the vaccination programme should also be provided.
Additional surveillance requirements for countries, zones or compartments declaring that they have regained freedom from avian influenza or from infection with high pathogenicity avian influenza viruses in poultry following an outbreak
In addition to the general conditions described in the above-mentioned articles, a Member Country declaring that it has regained country, zone or compartment freedom from avian influenza or from infection with high pathogenicity avian influenza viruses in poultry should show evidence of an active surveillance programme depending on the epidemiological circumstances of the outbreak to demonstrate the absence of the infection. This will require surveillance incorporating virus detection and antibody tests. The use of sentinel birds may facilitate the interpretation of surveillance results.
A Member Country declaring freedom of country, zone or compartment after an outbreak of avian influenza should report the results of an active surveillance programme in which the susceptible poultry population undergoes regular clinical examination and active surveillance planned and implemented in accordance with the general conditions and methods described in these recommendations. The surveillance should at least give the confidence that can be given by a randomised representative sample of the populations at risk.
Additional surveillance requirements for avian influenza free establishments
The declaration of avian influenza free establishments requires the demonstration of absence of infection with avian influenza viruses. Birds in these establishments should be randomly tested using virus detection or isolation tests, and serological methods, following the general conditions of these recommendations. The frequency of testing should be based on the risk of infection and at a maximum interval of 21 days.
The use and interpretation of serological and virus detection tests
Poultry infected
with avian influenza virus produce antibodies against haemagglutinin
(HA), neuraminidase (NA), nonstructural proteins (NSPs), nucleoprotein/matrix
(NP/M) and the polymerase complex proteins. Detection of antibodies
against the polymerase complex proteins is not covered in this chapter.
Tests for NP/M antibodies include direct and blocking ELISA, and
agar gel immunodiffusion (AGID) tests. Tests for antibodies against
NA include the neuraminidase inhibition (NI), indirect fluorescent
antibody and direct and blocking ELISA tests. For the HA, antibodies are
detected in haemagglutination inhibition (HI), ELISA and neutralisation
(SN) tests. The HI test is reliable in avian species but not in
mammals. The SN test can be used to detect subtype specific antibodies
against the haemagglutinin and is the preferred test for mammals
and some avian species. The AGID test is reliable for detection
of NP/M antibodies in chickens and turkeys, but not in other avian
species. As an alternative, blocking ELISA tests have been developed
to detect NP/M antibodies in all avian species.
The HI and NI tests can be used to subtype influenza A viruses into 16 haemagglutinin and 9 neuraminidase subtypes. Such information is helpful for epidemiological investigations and in categorization of influenza A viruses.
Poultry can be vaccinated with a variety of influenza A vaccines including inactivated whole virus vaccines, and haemagglutinin expression-based vaccines. Antibodies against the haemagglutinin confer subtype specific protection. Various strategies can be used to differentiate vaccinated from infected birds including serosurveillance in unvaccinated sentinel birds or specific serological tests in the vaccinated birds.
Influenza A virus infection of unvaccinated birds including sentinels is detected by antibodies against the NP/M, subtype specific HA or NA proteins, or NSP. Poultry vaccinated with inactivated whole virus vaccines containing a virus of the same H sub-type but with a different neuraminidase may be tested for field exposure by applying serological tests directed to the detection of antibodies against the NA of the field virus. For example, birds vaccinated with H7N3 in the face of a H7N1 epidemic may be differentiated from infected birds (DIVA) by detection of subtype specific NA antibodies of the N1 protein of the field virus. Alternatively, in the absence of DIVA, inactivated vaccines may induce low titres of antibodies against NSP and the titre in infected birds would be markedly higher. Encouraging results have been obtained experimentally with this system, but it has not yet been validated in the field. In poultry vaccinated with haemagglutinin expression-based vaccines, antibodies are detected against the specific HA, but not any of the other viral proteins. Infection is evident by antibodies against the NP/M or NSP, or the specific NA protein of the field virus.
All flocks with seropositive results should be investigated. Epidemiological and supplementary laboratory investigation results should document the status of avian influenza infection for each positive flock.
A confirmatory test should have a higher specificity than the screening test and sensitivity at least equivalent than that of the screening test.
Information should be provided on the performance characteristics and validation of tests used.
Procedure in case of positive test results if vaccination is used
In case of vaccinated populations, one has to
exclude the likelihood that positive test results are indicative
of virus circulation. To this end, the following procedure should
be followed in the investigation of positive serological test results
derived from surveillance conducted
on vaccinated poultry. The
investigation should examine all evidence that might confirm or
refute the hypothesis that the positive results to the serological
tests employed in the initial survey were not due to virus circulation.
All the epidemiological information should be substantiated, and
the results should be collated in the final report.
Knowledge of the type of vaccine used is crucial in developing a serological based strategy to differentiate infected from vaccinated animals.
Inactivated whole virus vaccines can use either homologous or heterologous neuraminidase subtypes between the vaccine and field strains. If poultry in the population have antibodies against NP/M and were vaccinated with inactivated whole virus vaccine, the following strategies should be applied:
sentinel birds should remain NP/M antibody negative. If positive for NP/M antibodies, indicating influenza A virus infection, specific HI tests should be performed to identify H5 or H7 virus infection;
if vaccinated with inactivated whole virus vaccine containing homologous NA to field virus, the presence of antibodies against NSP could be indicative of infection. Sampling should be initiated to exclude the presence of avian influenza virus by either virus isolation or detection of virus specific genomic material or proteins;
if vaccinated with inactivated whole virus vaccine containing heterologous NA to field virus, presence of antibodies against the field virus NA or NSP would be indicative of infection. Sampling should be initiated to exclude the presence of avian influenza virus by either virus isolation or detection of virus specific genomic material or proteins.
Haemagglutinin expression-based vaccines contain the HA protein or gene homologous to the HA of the field virus. Sentinel birds as described above can be used to detect avian influenza infection. In vaccinated or sentinel birds, the presence of antibodies against NP/M, NSP or field virus NA is indicative of infection. Sampling should be initiated to exclude the presence of avian influenza virus by either virus isolation or detection of virus specific genomic material or proteins.
Procedure in case of test results indicative
of infection with avian influenza viruses
The detection of antibodies indicative of an infection with
avian influenza virus in unvaccinated poultry should
result in the initiation of epidemiological and virological investigations
to determine if the infections are
due to low and high pathogenicity viruses.
Virological testing should be initiated in all
antibody-positive and at risk populations. The samples should be evaluated
for the presence of avian influenza virus, by virus isolation and
identification, or detection of influenza A specific proteins or
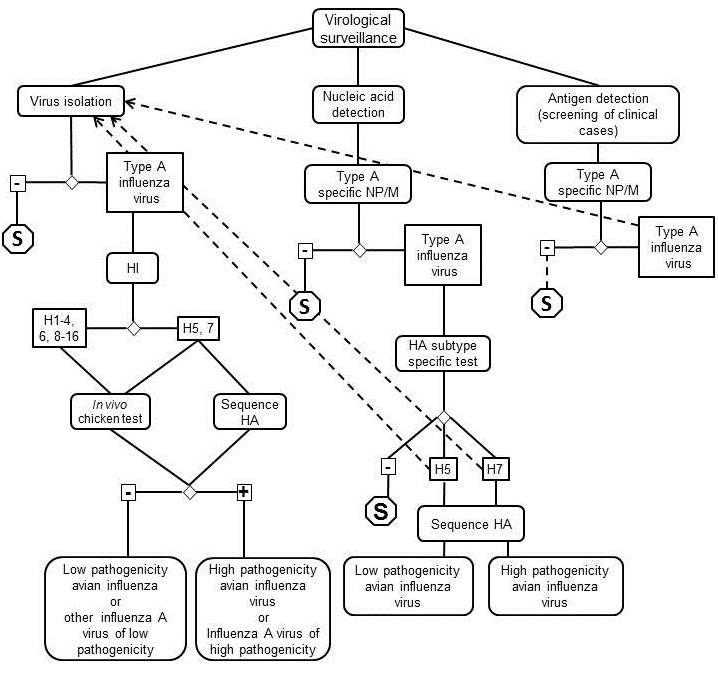
nucleic acids (Figure 2). Virus isolation is the gold standard for
detecting infection by avian influenza
virus. All influenza A virus isolates should be tested to determine
HA and NA subtypes, and in vivo tested in
chickens or sequencing of HA proteolytic cleavage site of H5 and
H7 subtypes for determination of classification as high or low pathogenicity
avian influenza viruses or other influenza A viruses. As an alternative,
nucleic acid detection tests have been developed and validated;
these tests have the sensitivity of virus isolation, but with the advantage
of providing results within a few hours. Samples with detection
of H5 and H7 HA subtypes by nucleic acid detection methods should
either be submitted for virus isolation, identification, and in vivo testing in chickens, or sequencing
of nucleic acids for determination of proteolytic cleavage site
as high or low pathogenicity avian influenza viruses. The use of
antigen detection systems, because of low sensitivity, should be
limited to screening clinical field cases for infection by
influenza A virus looking for NP/M proteins. NP/M positive samples
should be submitted for virus isolation, identification and pathogenicity
determination.
Laboratory results
should be examined in the context of the epidemiological situation.
Corollary information needed to complement the serological survey
and assess the possibility of viral circulation includes but is
not limited to:
characterisation of the existing production systems;
results of clinical surveillance of the suspects and their cohorts;
quantification of vaccinations performed on the affected sites;
sanitary protocol and history of the affected establishments;
control of animal identification and movements;
other parameters of regional significance
in historic avian influenza virus transmission.
The entire investigative process should be documented
as standard operating procedure within the epidemiological surveillance programme.
Figures 1 and 2 indicate the tests which are recommended for use in the investigation of poultryflocks.
| Abbreviations and acronyms: | |
| AGID | Agar gel immunodiffusion |
| DIVA | Differentiating infected from vaccinated animals |
| ELISA | Enzyme-linked immunosorbant assay |
| HA | Haemagglutinin |
| HI | Haemagglutination inhibition |
| NA | Neuraminidase |
| NP/M | Nucleoprotein and matrix protein |
| NSP | Nonstructural protein |
| S | No evidence of avian influenza virus |
Fig. 1. Schematic
representation of laboratory tests
for determining evidence
of avian influenza infection
through or following serological
surveys

Fig. 2. Schematic
representation of laboratory tests
for determining evidence
of avian influenza infection
using virological methods
nb: first adopted in 1998; most recent update adopted in 2017.
2018 ©OIE - Terrestrial Animal Health Code |