Terrestrial Animal Health Code
|
Terrestrial Animal Health Code |
Infection with foot and mouth disease virus
Many different species belonging to diverse taxonomic orders are known to be susceptible to infection with foot and mouth disease virus (FMDV). Their epidemiological significance depends upon the degree of susceptibility, the husbandry system, the density and extent of populations and the contacts between them. Amongst Camelidae, only Bactrian camels (Camelus bactrianus) are sufficiently susceptible to have potential for epidemiological significance. Dromedaries (Camelus dromedarius) are not susceptible to infection with FMDV while South American camelids are not considered to be of epidemiological significance.
For the purposes of the Terrestrial Code, foot and mouth disease (FMD) is defined as an infection of animals of the suborder ruminantia and of the family suidae of the order Artiodactyla, and Camelus bactrianus with FMDV.
The following defines the occurrence of infection with FMDV:
FMDV has been isolated from a sample from an animal listed in point 2; or
viral antigen or viral ribonucleic acid specific to FMDV has been identified in a sample from an animal listed in point 2, showing clinical signs consistent with FMD, or epidemiologically linked to a suspected or confirmed outbreak of FMD, or giving cause for suspicion of previous association or contact with FMDV; or
antibodies to structural or nonstructural proteins of FMDV, that are not a consequence of vaccination, have been identified in a sample from an animal listed in point 2, showing clinical signs consistent with FMD, or epidemiologically linked to a suspected or confirmed outbreak of FMD, or giving cause for suspicion of previous association or contact with FMDV.
Transmission of FMDV in a vaccinated population is demonstrated by change in virological or serological evidence indicative of recent infection, even in the absence of clinical signs.
For the purposes of the Terrestrial Code, the incubation period of FMD shall be 14 days.
Infection with FMDV can give rise to disease of variable severity and to FMDV transmission. FMDV may persist in the pharynx and associated lymph nodes of ruminants for a variable but limited period of time beyond 28 days. Such animals have been termed carriers. However, the only persistently infected species from which transmission of FMDV has been proven is the African buffalo (Syncerus caffer).
This chapter deals not only with the occurrence of clinical signs caused by FMDV, but also with the presence of infection with FMDV and transmission, in the absence of clinical signs.
Standards for diagnostic tests and vaccines are described in the Terrestrial Manual.
FMD free country or zone where vaccination is not practised
In defining a zone where vaccination is not practised the principles of Chapter 4.3. should be followed.
Susceptible animals in the FMD free country or zone where vaccination is not practised should be protected by the application of biosecurity measures that prevent the entry of FMDV into the free country or zone. Taking into consideration physical or geographical barriers with any neighbouring infected country or zone, these measures may include a protection zone.
To qualify for inclusion in the list of FMD free countries or zones where vaccination is not practised, a Member Country should:
have a record of regular and prompt animal disease reporting;
send a declaration to the OIE stating that during the past 12 months, within the proposed FMD free country or zone:
there has been no case of FMD;
no vaccination against FMD has been carried out;
supply documented evidence that for the past 12 months:
surveillance in accordance with Articles 8.8.40. to 8.8.42. has been implemented to detect clinical signs of FMD and demonstrate no evidence of:
infection with FMDV in unvaccinated animals;
FMDV transmission in previously vaccinated animals when the FMD free country or zone where vaccination is practised is seeking to become one where vaccination is not practised;
regulatory measures for the prevention and early detection of FMD have been implemented;
describe in detail and supply documented evidence that for the past 12 months the following have been properly implemented and supervised:
in the case of a FMD free zone, the boundaries of the proposed FMD free zone;
the boundaries and measures of a protection zone, if applicable;
the system for preventing the entry of FMDV into the proposed FMD free country or zone;
the control of the movement of susceptible animals, their meat and other products into the proposed FMD free country or zone, in particular the measures described in Articles 8.8.8., 8.8.9. and 8.8.12.;
no vaccinated animal has been introduced except in accordance with Articles 8.8.8. and 8.8.9.
The Member Country or the proposed free zone will be included in the list of FMD free countries or zones where vaccination is not practised only after the submitted evidence, based on the provisions of Article 1.6.6., has been accepted by the OIE.
Retention on the list requires that the information in points 2, 3 and 4 above be re-submitted annually and changes in the epidemiological situation or other significant events including those relevant to points 3b) and 4 should be reported to the OIE in accordance with the requirements in Chapter 1.1.
Provided the conditions of points 1 to 4 are fulfilled, the status of a country or zone will not be affected by applying official emergency vaccination to FMD susceptible animals in zoological collections in the face of a FMD threat identified by the Veterinary Authorities, provided that the following conditions are met:
the zoological collection has the primary purpose of exhibiting animals or preserving rare species, has been identified, including the boundaries of the facility, and is included in the country's contingency plan for FMD;
appropriate biosecurity measures are in place, including effective separation from other susceptible domestic populations or wildlife;
the animals are identified as belonging to the collection and any movements can be traced;
the vaccine used complies with the standards described in the Terrestrial Manual;
vaccination is conducted under the supervision of the Veterinary Authority;
the zoological collection is placed under surveillance for at least 12 months after vaccination.
In the event of the application for the status of a FMD free zone where vaccination is not practised to be assigned to a new zone adjacent to another FMD free zone where vaccination is not practised, it should be stated if the new zone is being merged with the adjacent zone to become one enlarged zone. If the two zones remain separate, details should be provided on the control measures to be applied for the maintenance of the status of the separate zones and particularly on the identification and the control of the movement of animals between the zones of the same status in accordance with Chapter 4.3.
FMD free country or zone where vaccination is practised
In defining a zone where vaccination is practised the principles of Chapter 4.3. should be followed.
Susceptible animals in the FMD free country or zone where vaccination is practised should be protected by the application of biosecurity measures that prevent the entry of FMDV into the free country or zone. Taking into consideration physical or geographical barriers with any neighbouring infected country or zone, these measures may include a protection zone.
Based on the epidemiology of FMD in the country, it may be decided to vaccinate only a defined subpopulation comprised of certain species or other subsets of the total susceptible population.
To qualify for inclusion in the list of FMD free countries or zones where vaccination is practised, a Member Country should:
have a record of regular and prompt animal disease reporting;
send a declaration to the OIE stating that, based on the surveillance described in point 3, within the proposed FMD free country or zone:
there has been no case of FMD during the past two years;
there has been no evidence of FMDV transmission during the past 12 months;
supply documented evidence that:
surveillance in accordance with Articles 8.8.40. to 8.8.42. has been implemented to detect clinical signs of FMD and demonstrate no evidence of:
infection with FMDV in unvaccinated animals;
FMDV transmission in vaccinated animals;
regulatory measures for the prevention and early detection of FMD have been implemented;
compulsory systematic vaccination in the target population has been carried out to achieve adequate vaccination coverage and population immunity;
vaccination has been carried out following appropriate vaccine strain selection;
describe in detail and supply documented evidence that the following have been properly implemented and supervised:
in case of FMD free zone, the boundaries of the proposed FMD free zone;
the boundaries and measures of a protection zone, if applicable;
the system for preventing the entry of FMDV into the proposed FMD free country or zone, in particular the measures described in Articles 8.8.8., 8.8.9. and 8.8.12.;
the control of the movement of susceptible animals and their products into the proposed FMD free country or zone.
The Member Country or the proposed free zone will be included in the list of FMD free countries or zones where vaccination is practised only after the submitted evidence, based on the provisions of Article 1.6.6., has been accepted by the OIE.
Retention on the list requires that the information in points 2, 3 and 4 above be re-submitted annually and changes in the epidemiological situation or other significant events including those relevant to points 3b) and 4 should be reported to the OIE in accordance with the requirements in Chapter 1.1.
If a Member Country that meets the requirements of a FMD free country or zone where vaccination is practised wishes to change its status to FMD free country or zone where vaccination is not practised, it should notify the OIE in advance of the intended date of cessation of vaccination and apply for the new status within 24 months of the cessation. The status of this country or zone remains unchanged until compliance with Article 8.8.2. is approved by the OIE. If the dossier for the new status is not provided within 24 months then the status of the country or zone as being free with vaccination will be suspended. If the country does not comply with requirements of Article 8.8.2., evidence should be provided within three months that it complies with Article 8.8.3. Otherwise the status will be withdrawn.
In the event of the application for the status of a FMD free zone where vaccination is practised to be assigned to a new zone adjacent to another FMD free zone where vaccination is practised, it should be stated if the new zone is being merged with the adjacent zone to become one enlarged zone. If the two zones remain separate, details should be provided on the control measures to be applied for the maintenance of the status of the separate zones and particularly on the identification and the control of the movement of animals between the zones of the same status in accordance with Chapter 4.3.
FMD free compartment
A FMD free compartment can be established in either a FMD free country or zone or in an infected country or zone. In defining such a compartment the principles of Chapters 4.3. and 4.4. should be followed. Susceptible animals in the FMD free compartment should be separated from any other susceptible animals by the application of an effective biosecurity management system.
A Member Country wishing to establish a FMD free compartment should:
have a record of regular and prompt animal disease reporting and, if not FMD free, have an official control programme and a surveillance system for FMD in place in accordance with Articles 8.8.40. to 8.8.42. that allows knowledge of the prevalence, distribution and characteristics of FMD in the country or zone;
declare for the FMD free compartment that:
there has been no case of FMD during the past 12 months;
no evidence of infection with FMDV has been found during the past 12 months;
vaccination against FMD is prohibited;
no animal vaccinated against FMD within the past 12 months is in the compartment;
animals, semen, embryos and animal products may only enter the compartment in accordance with relevant articles in this chapter;
documented evidence shows that surveillance in accordance with Articles 8.8.40. to 8.8.42. is in operation;
an animal identification and traceability system in accordance with Chapters 4.1. and 4.2. is in place;
describe in detail:
the animal subpopulation in the compartment;
the biosecurity plan to mitigate the risks identified by the surveillance carried out in accordance with point 1.
The compartment should be approved by the Veterinary Authority. The first approval should only be granted when no case of FMD has occurred within a ten-kilometre radius of the compartment during the past three months.
FMD infected country or zone
For the purposes of this chapter, a FMD infected country or zone is one that does not fulfil the requirements to qualify as either FMD free where vaccination is not practised or FMD free where vaccination is practised.
Establishment of a containment zone within a FMD free country or zone
In the event of limited outbreaks within a FMD free country or zone, including within a protection zone, with or without vaccination, a single containment zone, which includes all outbreaks, may be established for the purpose of minimising the impact on the entire country or zone.
For this to be achieved and for the Member Country to take full advantage of this process, the Veterinary Authority should submit as soon as possible to the OIE, in support of the application, documented evidence that:
on suspicion, a strict standstill has been imposed on the suspected establishments and in the country or zone animal movement control has been imposed and effective controls on the movement of other commodities mentioned in this chapter are in place;
on confirmation, an additional standstill of susceptible animals has been imposed in the entire containment zone and the movement controls described in point 1 have been reinforced;
the definitive boundaries of the containment zone have been established after an epidemiological investigation (trace-back, trace-forward) has demonstrated that the outbreaks are epidemiologically related and limited in number and geographic distribution;
investigations into the likely source of the outbreaks have been carried out;
a stamping-out policy, with or without the use of emergency vaccination, has been applied;
no new cases have been found in the containment zone within a minimum of two incubation periods as defined in Article 8.8.1. after the application of a stamping-out policy to the last detected case;
the susceptible domestic and captive wild animal populations within the containment zone are clearly identified as belonging to the containment zone;
surveillance in accordance with Articles 8.8.40. to 8.8.42. is in place in the containment zone and in the rest of the country or zone;
measures that prevent the spread of FMDV to the rest of the country or zone, taking into consideration physical and geographical barriers, are in place.
The free status of the areas outside the containment zone is suspended while the containment zone is being established. The free status of these areas may be reinstated irrespective of the provisions of Article 8.8.7., once the containment zone has been approved by the OIE as complying with points 1 to 9 above. Commodities from susceptible animals for international trade should be identified as to their origin, either from inside or outside the containment zone.
In the event of recurrence of infection with FMDV in unvaccinated animals or FMDV transmission in vaccinated animals in the containment zone, the approval of the containment zone is withdrawn and the FMD status of the whole country or zone is suspended until the relevant requirements of Article 8.8.7. are fulfilled.
The recovery of the FMD free status of the containment zone should be achieved within 12 months of its approval and follow the provisions of Article 8.8.7.
Recovery of free status (see Figures 1 and 2)
When a FMD case occurs in a FMD free country or zone where vaccination is not practised, one of the following waiting periods is required to regain this free status:
three months after the disposal of the last animal killed where a stamping-out policy, without emergency vaccination, and surveillance are applied in accordance with Articles 8.8.40. to 8.8.42.; or
three months after the disposal of the last animal killed or the slaughter of all vaccinated animals, whichever occurred last, where a stamping-out policy, emergency vaccination and surveillance in accordance with Articles 8.8.40. to 8.8.42. are applied; or
six months after the disposal of the last animal killed or the last vaccination whichever occurred last, where a stamping-out policy, emergency vaccination not followed by the slaughtering of all vaccinated animals, and surveillance in accordance with Articles 8.8.40. to 8.8.42. are applied. However, this requires a serological survey based on the detection of antibodies to nonstructural proteins of FMDV to demonstrate no evidence of infection in the remaining vaccinated population.
The country or zone will regain the status of FMD free country or zone where vaccination is not practised only after the submitted evidence, based on the provisions of Article 1.6.6., has been accepted by the OIE.
The time periods in points 1a) to 1c) are not affected if official emergency vaccination of zoological collections has been carried out following the relevant provisions of Article 8.8.2.
Where a stamping-out policy is not practised, the above waiting periods do not apply, and Article 8.8.2. applies.
When a FMD case occurs in a FMD free country or zone where vaccination is not practised, the following waiting period is required to gain the status of FMD free country or zone where vaccination is practised: six months after the disposal of the last animal killed where a stamping-out policy has been applied and a continued vaccination policy has been adopted, provided that surveillance is applied in accordance with Articles 8.8.40. to 8.8.42., and a serological survey based on the detection of antibodies to nonstructural proteins of FMDV demonstrates no evidence of FMDV transmission.
The country or zone can gain the status of FMD free country or zone where vaccination is practised only after the submitted evidence, based on the provisions of Article 1.6.6., has been accepted by the OIE.
Where a stamping-out policy is not practised, the above waiting periods do not apply, and Article 8.8.3. applies.
When a case of FMD occurs in a FMD free country or zone where vaccination is practised, one of the following waiting periods is required to regain this free status:
six months after the disposal of the last animal killed where a stamping-out policy, with emergency vaccination, and surveillance in accordance with Articles 8.8.40. to 8.8.42. are applied, provided that serological surveillance based on the detection of antibodies to nonstructural proteins of FMDV demonstrates no evidence of virus transmission; or
12 months after the detection of the last case where
a stamping-out policy is
not applied, but where emergency vaccination and surveillance in
accordance with Articles 8.8.40. to 8.8.42. are
applied, provided that serological surveillance based
on the detection of antibodies to nonstructural proteins of FMDV demonstrates
no evidence of virus transmission.
Where emergency vaccination is not applied, the above waiting periods do not apply, and Article 8.8.3. applies.
The country or zone will
regain the status of FMD free country or zone where vaccination is
practised only after the submitted evidence, based on the provisions
of Article 1.6.6.,
has been accepted by the OIE.
When a FMD case occurs in a FMD free compartment, Article 8.8.4. applies.
Member Countries applying for the recovery of status should do so only when the respective requirements for the recovery of status are met. When a containment zone has been established, the restrictions within the containment zone should be lifted in accordance with the requirements of this article only when the disease has been successfully eradicated within the containment zone.
For Member Countries not applying for recovery within 24 months after suspension, the provisions of Article 8.8.2., Article 8.8.3. or Article 8.8.4. apply.
Direct transfer of FMD susceptible animals from an infected zone for slaughter in a free zone (whether vaccination is practised or not)
In order not to jeopardise the status of a free zone, FMD susceptible animals should only leave the infected zone if transported directly to slaughter in the nearest designated slaughterhouse/abattoir under the following conditions:
no FMD susceptible animal has been introduced into the establishment of origin and no animal in the establishment of origin has shown clinical signs of FMD for at least 30 days prior to movement;
the animals were kept in the establishment of origin for at least three months prior to movement;
FMD has not occurred within a 10 kilometre radius of the establishment of origin for at least four weeks prior to movement;
the animals should be transported under the supervision of the Veterinary Authority in a vehicle, which was cleansed and disinfected before loading, directly from the establishment of origin to the slaughterhouse/abattoir without coming into contact with other susceptible animals;
such a slaughterhouse/abattoir is not approved for the export of fresh meat during the time it is handling the meat of animals from the infected zone;
vehicles and the slaughterhouse/abattoir should be subjected to thorough cleansing and disinfection immediately after use.
The animals should have been subjected to ante- and post-mortem inspection within 24 hours before and after slaughter with no evidence of FMD, and the meat derived from them treated in accordance with point 2 of Article 8.8.22. or Article 8.8.23. Other products obtained from the animals and any products coming into contact with them should be treated in accordance with Articles 8.8.31. to 8.8.38. in order to destroy any FMDV potentially present.
Direct transfer of FMD susceptible animals from a containment zone for slaughter in a free zone (whether vaccination is practised or not)
In order not to jeopardise the status of a free zone, FMD susceptible animals should only leave the containment zone if transported directly to slaughter in the nearest designated slaughterhouse/abattoir under the following conditions:
the containment zone has been officially established in accordance with the requirements in Article 8.8.6.;
the animals should be transported under the supervision of the Veterinary Authority in a vehicle, which was cleansed and disinfected before loading, directly from the establishment of origin to the slaughterhouse/abattoir without coming into contact with other susceptible animals;
such an slaughterhouse/abattoir is not approved for the export of fresh meat during the time it is handling the meat of animals from the containment zone;
vehicles and the slaughterhouse/abattoir should be subjected to thorough cleansing and disinfection immediately after use.
The animals should have been subjected to ante- and post-mortem inspection within 24 hours before and after slaughter with no evidence of FMD and the meat derived from them treated in accordance with point 2 of Article 8.8.22. or Article 8.8.23. Other products obtained from the animals and any products coming into contact with them should be treated in accordance with Articles 8.8.31. to 8.8.38. in order to destroy any FMDV potentially present.
Recommendations for importation from FMD free countries or zones where vaccination is not practised or FMD free compartments
For FMD susceptible animals
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that the animals:
showed no clinical sign of FMD on the day of shipment;
were kept since birth or for at least the past three months in a FMD free country or zone where vaccination is not practised or a FMD free compartment;
if transiting an infected zone, were not exposed to any source of FMDV during transportation to the place of shipment.
Recommendations for importation from FMD free countries or zones where vaccination is practised
For domestic ruminants and pigs
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that the animals:
showed no clinical sign of FMD on the day of shipment;
were kept since birth or for at least the past three months in a FMD free country or zone where vaccination is practised;
were subjected to a test for FMD with negative results;
if transiting an infected zone, were not exposed to any source of FMDV during transportation to the place of shipment.
Recommendations for importation from FMD infected countries or zones where an official control programme exists
For domestic ruminants and pigs
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the animals showed no clinical sign of FMD on the day of shipment;
prior to isolation, the animals were kept in the establishment of origin:
for 30 days, or since birth if younger than 30 days, if a stamping-out policy is applied to control FMD in the exporting country or zone, or
for three months, or since birth if younger than three months if a stamping-out policy is not applied to control FMD in the exporting country or zone;
FMD has not occurred within the establishment of origin for the relevant period as defined in points 2a) and 2b) above;
the animals were isolated in an establishment for the 30 days prior to shipment, and all animals in isolation were subjected to diagnostic virological and serological tests for evidence of FMDV with negative results on samples collected at least 28 days after the start of isolation period, and that FMD did not occur within a 10 kilometre radius of the establishment during that period, or the establishment is a quarantine station;
the animals were not exposed to any source of FMDV during their transportation from the establishment to the place of shipment.
Recommendations for importation from FMD free countries or zones where vaccination is not practised or FMD free compartments
For fresh semen of domestic ruminants and pigs
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the donor males:
showed no clinical sign of FMD on the day of collection of the semen;
were kept for at least three months prior to collection in a FMD free country or zone where vaccination is not practised or FMD free compartments;
were kept in an artificial insemination centre where none of the animals had a history of infection with FMDV;
the semen was collected, processed and stored in accordance with Chapters 4.5. and 4.6.
Recommendations for importation from FMD free countries or zones where vaccination is not practised or FMD free compartments
For frozen semen of domestic ruminants and pigs
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the donor males:
showed no clinical sign of FMD on the day of collection of the semen and for the following 30 days;
were kept for at least three months prior to collection in a FMD free country or zone where vaccination is not practised or FMD free compartments;
the semen was collected, processed and stored in accordance with Chapters 4.5. and 4.6.
Recommendations for importation from FMD free countries or zones where vaccination is practised
For frozen semen of domestic ruminants and pigs
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the donor males:
showed no clinical sign of FMD on the day of collection of the semen and for the following 30 days;
were kept for at least three months prior to collection in a FMD free country or zone where vaccination is practised;
either
have been vaccinated at least twice, with the last vaccination not less than one month and not more than six months prior to collection, unless protective immunity has been demonstrated for more than six months;
or
were subjected, not less than 21 days after collection of the semen, to tests for antibodies against FMDV, with negative results;
the semen:
was collected, processed and stored in accordance with Chapters 4.5. and 4.6.;
was stored in the country of origin for a period of at least one month following collection, and during this period no animal on the establishment where the donor animals were kept showed any sign of FMD.
Recommendations for importation from FMD infected countries or zones
For frozen semen of domestic ruminants and pigs
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the donor males:
showed no clinical sign of FMD on the day of collection of the semen and for the following 30 days;
were kept in an artificial insemination centre where no animal had been added in the 30 days before collection, and that FMD has not occurred within a 10 kilometre radius of the artificial insemination centre for the 30 days before and after collection;
either
have been vaccinated at least twice, with the last vaccination not less than one month and not more than six months prior to collection, unless protective immunity has been demonstrated for more than six months;
or
were subjected, not less than 21 days after collection of the semen, to tests for antibodies against FMDV, with negative results;
the semen:
was collected, processed and stored in accordance with Chapters 4.5. and 4.6.;
was subjected, with negative results, to a test for evidence of FMDV if the donor male has been vaccinated within the 12 months prior to collection;
was stored in the country of origin for a period of at least one month following collection, and that during this period no animal on the establishment where the donor males were kept showed any sign of FMD.
Recommendations for the importation of in vivo derived embryos of cattle
Irrespective of the FMD status of the exporting country, zone or compartment, Veterinary Authorities should authorise without restriction on account of FMD the import or transit through their territory of in vivo derived embryos of cattle subject to the presentation of an international veterinary certificate attesting that the embryos were collected, processed and stored in accordance with Chapters 4.7. and 4.9., as relevant.
Recommendations for importation from FMD free countries or zones where vaccination is not practised or FMD free compartments
For in vitro produced embryos of cattle
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the donor females:
showed no clinical sign of FMD at the time of collection of the oocytes;
were kept for at least three months prior to collection in a FMD free country or zone where vaccination is not practised or FMD free compartments;
fertilisation was achieved with semen meeting the conditions referred to in Articles 8.8.13., 8.8.14., 8.8.15. or 8.8.16., as relevant;
the oocytes were collected, and the embryos were processed and stored in accordance with Chapters 4.8. and 4.9., as relevant.
Recommendations for importation from FMD free countries or zones where vaccination is practised
For in vitro produced embryos of cattle
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the donor females:
showed no clinical sign of FMD at the time of collection of the oocytes;
were kept for at least three months prior to collection in a FMD free country or zone where vaccination is practised;
either
have been vaccinated at least twice, with the last vaccination not less than one month and not more than six months prior to collection, unless protective immunity has been demonstrated for more than six months;
or
were subjected, not less than 21 days after collection, to tests for antibodies against FMDV, with negative results;
fertilisation was achieved with semen meeting the conditions referred to in Articles 8.8.13., 8.8.14., 8.8.15. or 8.8.16., as relevant;
the oocytes were collected, and the embryos were processed and stored in accordance with Chapters 4.8. and 4.9., as relevant.
Recommendations for importation from FMD free countries or zones where vaccination is not practised or FMD free compartments
For fresh meat or meat products of FMD susceptible animals
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that the entire consignment of meat comes from animals which:
have been kept in a FMD free country or zone where vaccination is not practised or FMD free compartment, or which have been imported in accordance with Article 8.8.10., Article 8.8.11. or Article 8.8.12.;
have been slaughtered in an approved slaughterhouse/abattoir and have been subjected to ante- and post-mortem inspections with favourable results.
Recommendations for importation from FMD free countries or zones where vaccination is practised
For fresh meat and meat products of ruminants and pigs
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that the entire consignment of meat comes from animals which:
have been kept in the FMD free country or zone where vaccination is practised, or which have been imported in accordance with Article 8.8.10., Article 8.8.11. or Article 8.8.12.;
have been slaughtered in an approved slaughterhouse/abattoir and have been subjected to ante- and post-mortem inspections for FMD with favourable results;
for ruminants the head, including the pharynx, tongue and associated lymph nodes, has been excluded from the shipment.
Recommendations for importation from FMD infected countries or zones where an official control programme exists
For fresh meat of cattle and water buffaloes (Bubalus bubalis) (excluding feet, head and viscera)
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that the entire consignment of meat:
comes from animals which:
have remained, for at least three months prior to slaughter, in a zone of the exporting country where cattle and water buffaloes are regularly vaccinated against FMD and where an official control programme is in operation;
have been vaccinated at least twice with the last vaccination not more than six months, unless protective immunity has been demonstrated for more than six months, and not less than one month prior to slaughter;
were kept for the past 30 days in an establishment, and that FMD has not occurred within a 10 kilometre radius of the establishment during that period, or the establishment is a quarantine station;
have been transported, in a vehicle which was cleansed and disinfected before the cattle and water buffaloes were loaded, directly from the establishment of origin or quarantine station to the approved slaughterhouse/abattoir without coming into contact with other animals which do not fulfil the required conditions for export;
have been slaughtered in an approved slaughterhouse/abattoir:
which is officially designated for export;
in which no FMD has been detected during the period between the last disinfection carried out before slaughter and the shipment for export has been dispatched;
have been subjected to ante- and post-mortem inspections within 24 hours before and after slaughter with no evidence of FMD;
comes from deboned carcasses:
from which the major lymphatic nodes have been removed;
which, prior to deboning, have been submitted to maturation at a temperature greater than + 2°C for a minimum period of 24 hours following slaughter and in which the pH value was less than 6.0 when tested in the middle of both the longissimus dorsi muscle.
Recommendations for importation from FMD infected countries or zones
For meat products of FMD susceptible animals
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
the entire consignment of meat products come from animals which have been slaughtered in an approved slaughterhouse/abattoir and have been subjected to ante- and post-mortem inspections for FMD with favourable results;
the meat products have been processed to ensure the destruction of FMDV in accordance with one of the procedures in Article 8.8.31.;
the necessary precautions were taken after processing to avoid contact of the meat products with any potential source of FMDV.
Recommendations for importation from FMD free countries or zones where vaccination either is or is not practised or FMD free compartments
For milk and milk products intended for human consumption and for products of animal origin (from FMD susceptible animals) intended for use in animal feeding or for agricultural or industrial use
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that these products come from animals which have been kept in a FMD free country, zone or compartment, or which have been imported in accordance with Article 8.8.10., Article 8.8.11. or Article 8.8.12.
Recommendations for importation from FMD infected countries or zones where an official control programme exists
For milk and milk products
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
these products:
originate from establishments which were not infected or suspected of being infected with FMD at the time of milk collection;
have been processed to ensure the destruction of FMDV in accordance with one of the procedures in Article 8.8.35. and in Article 8.8.36.;
the necessary precautions were taken after processing to avoid contact of the products with any potential source of FMDV.
Recommendations for importation from FMD infected countries
For blood-meal and meat-meals from FMD susceptible animals
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that the manufacturing method for these products included heating to a minimum core temperature of 70°C for at least 30 minutes.
Recommendations for importation from FMD infected countries
For wool, hair, bristles, raw hides and skins from FMD susceptible animals
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that:
these products have been processed to ensure the destruction of FMDV in accordance with one of the procedures in Articles 8.8.32., 8.8.33. and 8.8.34.;
the necessary precautions were taken after collection or processing to avoid contact of the products with any potential source of FMDV.
Veterinary Authorities should authorise, without restriction, the import or transit through their territory of semi-processed hides and skins (limed hides, pickled pelts, and semi-processed leather such as wet blue and crust leather), provided that these products have been submitted to the usual chemical and mechanical processes in use in the tanning industry.
Recommendations for importation from FMD infected countries or zones
For straw and forage
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that these commodities:
are free of grossly identified contamination with material of animal origin;
have been subjected to one of the following treatments, which, in the case of material sent in bales, has been shown to penetrate to the centre of the bale:
either to the action of steam in a closed chamber such that the centre of the bales has reached a minimum temperature of 80°C for at least ten minutes,
or to the action of formalin fumes (formaldehyde gas) produced by its commercial solution at 35-40% in a chamber kept closed for at least eight hours and at a minimum temperature of 19°C;
OR
have been kept in bond for at least four months before being released for export.
Recommendations for importation from FMD free countries or zones where vaccination either is or is not practised
For skins and trophies derived from FMD susceptible wildlife
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that these products are derived from animals that have been killed in such a country or zone or which have been imported from a country, zone or compartment free from FMD.
Recommendations for importation from FMD infected countries or zones
For skins and trophies derived from FMD susceptible wildlife
Veterinary Authorities should require the presentation of an international veterinary certificate attesting that these products have been processed to ensure the destruction of FMDV in accordance with the procedures in Article 8.8.37.
Procedures for the inactivation of FMDV in meat and meat products
For the inactivation of FMDV present in meat and meat products, one of the following procedures should be used:
Canning
Meat and meat products are subjected to heat treatment in a hermetically sealed container to reach an internal core temperature of at least 70°C for a minimum of 30 minutes or to any equivalent treatment which has been demonstrated to inactivate FMDV.
Thorough cooking
Meat, previously deboned and defatted, and meat products are subjected to a heat treatment that results in a core temperature of at least 70°C for a minimum of 30 minutes.
After cooking, they should be packed and handled in such a way they are not exposed to a source of FMDV.
Drying after salting
When rigor mortis is complete, the meat is deboned, treated with salt (NaCl) and ’completely dried’. It should not deteriorate at ambient temperature.
’Completely dried' is defined as a moisture protein ratio that is not greater than 2.25:1 or a water activity (Aw) that is not greater than 0.85.
Procedures for the inactivation of FMDV in wool and hair
For the inactivation of FMDV present in wool and hair for industrial use, one of the following procedures should be used:
industrial washing, which consists of the immersion of the wool in a series of baths of water, soap and sodium hydroxide (soda) or potassium hydroxide (potash);
chemical depilation by means of slaked lime or sodium sulphide;
fumigation with formaldehyde in a hermetically sealed chamber for at least 24 hours;
industrial scouring which consists of the immersion of wool in a water-soluble detergent held at 60-70°C;
storage of wool at 4°C for four months, 18°C for four weeks or 37°C for eight days.
Procedures for the inactivation of FMDV in bristles
For the inactivation of FMDV present in bristles for industrial use, one of the following procedures should be used:
boiling for at least one hour; or
immersion for at least 24 hours in a 1% aqueous solution of formaldehyde.
Procedures for the inactivation of FMDV in raw hides and skins
For the inactivation of FMDV present in raw hides and skins for industrial use, the following procedure should be used: treatment for at least 28 days with salt (NaCl) containing 2% sodium carbonate (Na2CO3).
Procedures for the inactivation of FMDV in milk and cream for human consumption
For the inactivation of FMDV present in milk and cream for human consumption, one of the following procedures should be used:
a process applying a minimum temperature of 132°C for at least one second (ultra-high temperature [UHT]), or
if the milk has a pH less than 7.0, a process applying a minimum temperature of 72°C for at least 15 seconds (high temperature - short time pasteurisation [HTST]), or
if the milk has a pH of 7.0 or greater, the HTST process applied twice.
Procedures for the inactivation of FMDV in milk for animal consumption
For the inactivation of FMDV present in milk for animal consumption, one of the following procedures should be used:
the HTST process applied twice; or
HTST combined with another physical treatment, e.g. maintaining a pH 6 for at least one hour or additional heating to at least 72°C combined with desiccation; or
UHT combined with another physical treatment referred to in point 2 above.
Procedures for the inactivation of FMDV in skins and trophies from wildlife susceptible to the disease
For the inactivation of FMDV present in skins and trophies from wild animals susceptible to FMD, one of the following procedures should be used prior to complete taxidermal treatment:
boiling in water for an appropriate time so as to ensure that any matter other than bone, horns, hooves, claws, antlers or teeth is removed; or
gamma irradiation at a dose of at least 20 kilogray at room temperature (20°C or higher); or
soaking, with agitation, in a 4% (weight/volume) solution of sodium carbonate (Na2CO3) maintained at pH 11.5 or greater for at least 48 hours; or
soaking, with agitation, in a formic acid solution (100 kg salt [NaCl] and 12 kg formic acid per 1,000 litres water) maintained at pH less than 3.0 for at least 48 hours; wetting and dressing agents may be added; or
in the case of raw hides, treating for at least 28 days with salt (NaCl) containing 2% sodium carbonate (Na2CO3).
Procedures for the inactivation of FMDV in casings of ruminants and pigs
For the inactivation of FMDV present in casings of ruminants and pigs, the following procedures should be used: treating for at least 30 days either with dry salt (NaCl) or with saturated brine (NaCl, aw< 0.80), or with phosphate supplemented salt containing 86.5% NaCl, 10.7% Na2HPO4 and 2.8% Na3PO4 (weight/weight/weight), either dry or as a saturated brine (aw< 0.80), and kept at a temperature of greater than 12°C during this entire period.
OIE endorsed official control programme for FMD
The overall objective of an OIE endorsed official control programme for FMD is for countries to progressively improve the situation and eventually attain FMD free status. The official control programme should be applicable to the entire country even if certain measures are directed towards defined subpopulations only.
Member Countries may, on a voluntary basis, apply for endorsement of their official control programme for FMD when they have implemented measures in accordance with this article.
For a Member Country's official control programme for FMD to be endorsed by the OIE, the Member Country should:
have a record of regular and prompt animal disease reporting in accordance with the requirements in Chapter 1.1.;
submit documented evidence of the capacity of the Veterinary Services to control FMD; one way of providing this evidence is through the OIE PVS Pathway;
submit a detailed plan of the programme to control and eventually eradicate FMD in the country or zone including:
the timeline;
the performance indicators for assessing the efficacy of the control measures to be implemented;
documentation indicating that the official control programme for FMD is applicable to the entire country;
submit a dossier on the epidemiology of FMD in the country describing the following:
the general epidemiology in the country highlighting the current knowledge and gaps and the progress that has been made in controlling FMD;
the measures implemented to prevent introduction of infection, the rapid detection of, and response to, all FMD outbreaks in order to reduce the incidence of FMD outbreaks and to eliminate FMDV transmission in at least one zone in the country;
the main livestock production systems and movement patterns of FMD susceptible animals and their products within and into the country;
submit evidence that FMD surveillance is in place:
taking into account provisions in Chapter 1.4. and the provisions on surveillance of this chapter;
have diagnostic capability and procedures, including regular submission of samples to a laboratory that carries out diagnosis and further characterisation of strains;
where vaccination is practised as a part of the official control programme for FMD, provide:
evidence (such as copies of legislation) that vaccination of selected populations is compulsory;
detailed information on vaccination campaigns, in particular on:
target populations for vaccination;
monitoring of vaccination coverage, including serological monitoring of population immunity;
technical specification of the vaccines used, including matching with the circulating FMDV strains, and description of the licensing procedures in place;
the proposed timeline for the transition to the use of vaccines fully compliant with the standards and methods described in the Terrestrial Manual;
provide an emergency preparedness and response plan to be implemented in case of outbreaks.
The Member Country's official control programme for FMD will be included in the list of programmes endorsed by the OIE only after the submitted evidence, based on the provisions of Article 1.6.11., has been accepted by the OIE. Retention on the list requires an annual update on the progress of the official control programme and information on significant changes concerning the points above. Changes in the epidemiological situation and other significant events should be reported to the OIE in accordance with the requirements in Chapter 1.1.
The OIE may withdraw the endorsement of the official control programme if there is evidence of:
non-compliance with the timelines or performance indicators of the programme; or
significant problems with the performance of the Veterinary Services; or
an increase in the incidence of FMD that cannot be addressed by the programme.
General principles of surveillance
Articles 8.8.40. to 8.8.42. define the principles and provide a guide for the surveillance of FMD in accordance with Chapter 1.4. applicable to Member Countries seeking establishment, maintenance or recovery of freedom from FMD at the country, zone or compartment level or seeking endorsement by the OIE of their official control programme for FMD, in accordance with Article 8.8.39.Surveillance aimed at identifying disease and FMDV infection or transmission should cover domestic and, where appropriate, wildlife species as indicated in point 2 of Article 8.8.1.
Early detection
A surveillance system in accordance with Chapter 1.4. should be the responsibility of the Veterinary Authority and should provide an early warning system to report suspected cases throughout the entire production, marketing and processing chain. A procedure should be in place for the rapid collection and transport of samples to a laboratory for FMD diagnosis. This requires that sampling kits and other equipment be available to those responsible for surveillance. Personnel responsible for surveillance should be able to seek assistance from a team with expertise in FMD diagnosis and control.
Demonstration of freedom
The impact and epidemiology of FMD widely differ in different regions of the world and therefore it is inappropriate to provide specific recommendations for all situations. Surveillance strategies employed for demonstrating freedom from FMD in the country, zone or compartment at an acceptable level of confidence should be adapted to the local situation. For example, the approach to demonstrating freedom from FMD following an outbreak caused by a pig-adapted strain of FMDV should differ significantly from an approach designed to demonstrate freedom from FMD in a country or zone where African buffaloes (Syncerus caffer) provide a potential reservoir of infection.
Surveillance for FMD should be in the form of a continuing programme. Programmes to demonstrate no evidence of infection with FMDV and transmission should be carefully designed and implemented to avoid producing results that are insufficient to be accepted by the OIE or trading partners, or being excessively costly and logistically complicated.
The strategy and design of the surveillance programme will depend on the historical epidemiological circumstances including whether or not vaccination has been used.
A Member Country wishing to substantiate FMD freedom where vaccination is not practised should demonstrate no evidence of infection with FMDV.
A Member Country wishing to substantiate FMD freedom where vaccination is practised should demonstrate that FMDV has not been transmitted in any susceptible populations. Within vaccinated populations, serological surveys to demonstrate no evidence of FMDV transmission should target animals that are less likely to show vaccine-derived antibodies to nonstructural proteins, such as young animals vaccinated a limited number of times, or unvaccinated animals. In any unvaccinated subpopulation, surveillance should demonstrate no evidence of infection with FMDV.
Surveillance strategies employed for establishing and maintaining a compartment should identify the prevalence, distribution and characteristics of FMD outside the compartment.
OIE endorsed official control programme
Surveillance strategies employed in support of an OIE endorsed official control programme should demonstrate evidence of the effectiveness of any vaccination used and of the ability to rapidly detect all FMD outbreaks.
Therefore considerable latitude is available to Member Countries to design and implement surveillance to establish that the whole territory or part of it is free from FMDV infection and transmission and to understand the epidemiology of FMD as part of the official control programme.
The Member Country should submit a dossier to the OIE in support of its application that not only explains the epidemiology of FMD in the region concerned but also demonstrates how all the risk factors, including the role of wildlife, if appropriate, are identified and managed. This should include provision of scientifically based supporting data.
Surveillance strategies
The strategy employed to establish the prevalence
of infection with
FMDV or to substantiate freedom from FMDV infection or
transmission may be based on randomised or targeted clinical investigation
or sampling at an acceptable level of statistical confidence, as
described in Articles 1.4.4. and 1.4.5. If
an increased likelihood of infection in particular
localities or species can be identified, targeted sampling may be
appropriate. Clinical inspection may be targeted at particular species
likely to exhibit clear clinical signs (e.g. cattle and pigs). The Member
Country should justify the surveillance strategy
chosen and the frequency of sampling as adequate to detect the presence
of FMDV infection or transmission
in accordance with Chapter 1.4. and
the epidemiological situation.
The design of the sampling strategy should incorporate
an epidemiologically appropriate design prevalence. The sample size
selected for testing should be adequate to detect infection or
transmission if it were to occur at a predetermined minimum rate.
The sample size and expected disease prevalence
determine the level of confidence in the results of the survey.
The Member Country should justify the choice of design prevalence
and confidence level based on the objectives of surveillance and
the prevailing or historical epidemiological situation, in accordance with
Chapter 1.4.
Follow-up of suspected cases and interpretation
of results
An effective surveillance system will identify suspected cases that require immediate follow-up and investigation to confirm or exclude that the cause of the condition is FMDV. Samples should be taken and submitted for diagnostic testing, unless the suspected case can be confirmed or ruled out by epidemiological and clinical investigation. Details of the occurrence of suspected cases and how they were investigated and dealt with should be documented. This should include the results of diagnostic testing and the control measures to which the animals concerned were subjected during the investigation.
The sensitivity and specificity of the diagnostic
tests employed, including the performance of confirmatory tests,
are key factors in the design, sample size determination and interpretation
of the results obtained. The sensitivity and specificity of the
tests used should be validated for the vaccination or infection history
and production class of animals in the target population.
The surveillance design
should anticipate the occurrence of false positive reactions. If
the characteristics of the testing system are known, the rate at
which these false positives are likely to occur can be calculated
in advance. There should be an effective procedure for following-up
positives to determine with a high level of confidence, whether
or not they are indicative of infection or transmission.
This should involve supplementary tests and follow-up investigation
to collect diagnostic material from the original epidemiological unit and herds which
may be epidemiologically linked to it.
Laboratory results
should be examined in the context of the epidemiological situation.
Corollary information needed to complement the serological survey
and assess the possibility of viral transmission includes but is
not limited to:
characterisation of the existing production systems;
results of clinical surveillance of
the suspects and their cohorts;
description of number of, and protocol for, vaccinations performed
in the area under assessment;
biosecurity and
history of the establishments with
reactors;
identification and traceability of animals
and control of their movements;
other parameters of regional significance in historic FMDV transmission.
Demonstration of population immunity
Following routine vaccination, evidence should be provided to demonstrate the effectiveness of the vaccination programme such as adequate vaccination coverage and population immunity. This can help to reduce reliance on post-vaccination surveys for residual infection and transmission.
In designing serological surveys to estimate population immunity, blood sample collection should be stratified by age to take account of the number of vaccinations the animals have received. The interval between last vaccination and sampling depends upon the intended purpose. Sampling at one or two months after vaccination provides information on the efficiency of the vaccination programme, while sampling before or at the time of revaccination provides information on the duration of immunity. When multivalent vaccines are used, tests should be carried out to determine the antibody level at least for each serotype, if not for each antigen blended into the vaccine. The test cut-off for an acceptable level of antibody should be selected with reference to protective levels demonstrated by vaccine-challenge test results for the antigen concerned. Where the threat from circulating virus has been characterised as resulting from a field virus with significantly different antigenic properties from the vaccine virus, this should be taken into account when interpreting the protective effect of population immunity. Figures for population immunity should be quoted with reference to the total of susceptible animals in a given subpopulation and in relation to the subset of vaccinated animals.
The entire investigative process should be documented within the surveillance programme.
All the epidemiological information should be substantiated, and the results should be collated in the final report.
Methods of surveillance
Clinical surveillance
Farmers and workers who have day-to-day contact
with livestock, as well as veterinary para-professionals, veterinarians and
diagnosticians, should report promptly any suspicion of FMD. The Veterinary Authority should implement
programmes to raise awareness among them.
Clinical surveillance requires the physical examination of susceptible animals. Although significant emphasis is placed on the diagnostic value of mass serological screening, surveillance based on clinical inspection may provide a high level of confidence of detection of disease if a sufficient number of clinically susceptible animals is examined at an appropriate frequency and investigations are recorded and quantified.
Clinical examination and diagnostic testing should be applied to clarify the status of suspected cases. Diagnostic testing may confirm clinical suspicion, while clinical surveillance may contribute to confirmation of positive laboratory test results. Clinical surveillance may be insufficient in wildlife and domestic species that usually do not show clinical signs or husbandry systems that do not permit sufficient observations. In such situations, serological surveillance should be used. Hunting, capture and non-invasive sampling and observation methods can be used to obtain information and diagnostic samples from wildlife species.
Virological surveillance
Establishment of the molecular, antigenic and
other biological characteristics of the causative virus, as well
as its source, is mostly dependent upon clinical surveillance to
provide samples. FMDV isolates should be sent regularly to an OIE
Reference Laboratory.
Virological surveillance aims to:
confirm clinically suspected cases;
follow up positive serological results;
characterise isolates for epidemiological studies and vaccine matching;
monitor populations at risk for the presence and transmission of the virus.
Serological surveillance
Serological surveillance aims
to detect antibodies resulting from infection or vaccination using
nonstructural protein tests or structural protein tests.
Serological surveillance may
be used to:
estimate the prevalence or substantiate freedom from FMDV infection or transmission;
monitor population immunity.
Serum collected for other purposes can be used
for FMD surveillance,
provided the principles of survey design described in this chapter
are met.
The results of random or targeted serological surveys are important in providing reliable evidence of the FMD situation in a country, zone or compartment. It is therefore essential that the survey be thoroughly documented.
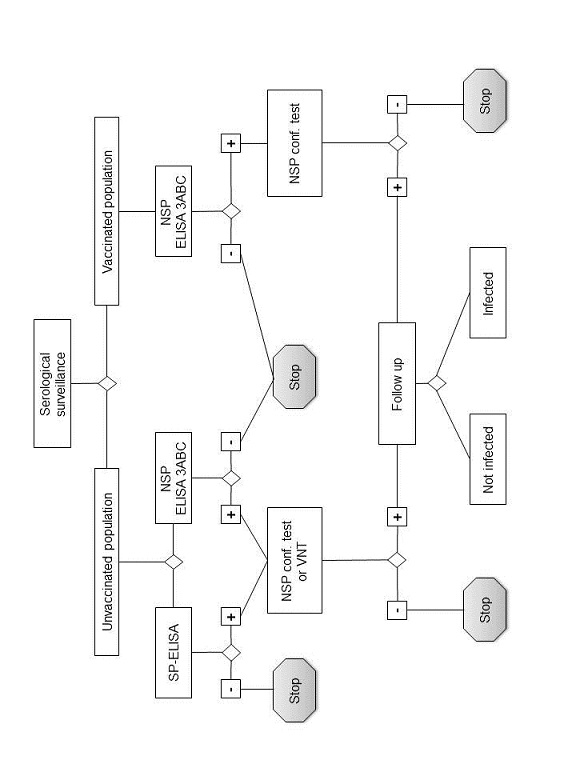
The use and interpretation of serological tests (see Figure 3)
The selection and interpretation of serological tests should be considered in the context of the epidemiological situation. Test protocols, reagents, performance characteristics and validation of all tests used should be known. Where combinations of tests are used, the overall test system performance characteristics should also be known.
Animals infected with FMDV produce antibodies
to both the structural proteins and the nonstructural proteins of
the virus. Vaccinated animals produce antibodies mainly or entirely
to the structural proteins of the virus depending upon vaccine purity.
The structural protein tests are serotype specific and for optimal
sensitivity one should select an antigen or virus closely related
to the field strain expected. In unvaccinated populations, structural
protein tests may be used to screen sera for evidence of FMDV infection or
transmission or to detect the introduction of vaccinated animals.
In vaccinated populations, structural protein tests may be used
to monitor the serological response to the vaccination.
Nonstructural protein tests may be used to screen sera for evidence of infection or transmission of all serotypes of FMDV regardless of the vaccination status of the animals provided the vaccines comply with the standards of the Terrestrial Manual with respect to purity. However, although animals vaccinated and subsequently infected with FMDV develop antibodies to nonstructural proteins, the levels may be lower than those found in infected animals that have not been vaccinated. To ensure that all animals that had contact with FMDV have seroconverted, it is recommended that for each vaccination area samples for nonstructural protein antibody testing are taken not earlier than 30 days after the last case and in any case not earlier than 30 days after the last vaccination.
Positive FMDV antibody test results can have
four possible causes:
infection with
FMDV;
vaccination against
FMD;
maternal antibodies (maternal antibodies in cattle are usually found only up to six months of age but in some individuals and in some other species, maternal antibodies can be detected for longer periods);
non-specific reactivity of the serum in the tests used.
Procedure in case of positive test results
The proportion and strength of seropositive reactors
should be taken into account when deciding if they are laboratory confirmed
reactors or further investigation and testing are required.
When false positive results are suspected, seropositive reactors should be retested in the laboratory using repeat and confirmatory tests. Tests used for confirmation should be of high diagnostic specificity to minimise false positive test results. The diagnostic sensitivity of the confirmatory test should approach that of the screening test.
All herds with at least one laboratory confirmed reactor should be investigated. The investigation should examine all evidence, which may include the results of virological tests and of any further serological tests that might confirm or refute the hypothesis that the positive results to the serological tests employed in the initial survey were due to FMDV transmission. This investigation should document the status for each positive herd. Epidemiological investigation should be continued concurrently.
Clustering of seropositive results within herds or within a region should be investigated as it may reflect any of a series of events, including the demographics of the population sampled, vaccinal exposure or the presence of infection or transmission. As clustering may signal infection or transmission, the investigation of all instances should be incorporated in the survey design.
Paired serology can be used to identify FMDV transmission by demonstrating an increase in the number of seropositive animals or an increase in antibody titre at the second sampling.
The investigation should include the reactor animals, susceptible animals of the same epidemiological unit and susceptible animals that have been in contact or otherwise epidemiologically associated with the reactor animals. The animals sampled should remain in the establishment pending test results, should be clearly identified, accessible and should not be vaccinated during the investigations, so that they can be retested after an appropriate period of time. Following clinical examination, a second sample should be taken, after an appropriate time has lapsed, from the animals tested in the initial survey with emphasis on animals in direct contact with the reactors. If the animals are not individually identified, a new serological survey should be carried out in the establishments after an appropriate time, repeating the application of the primary survey design. If FMDV is not circulating, the magnitude and prevalence of antibody reactivity observed should not differ in a statistically significant manner from that of the primary sample.
In some circumstances, unvaccinated sentinel animals may also be used. These can be young animals from unvaccinated dams or animals in which maternally conferred immunity has lapsed and preferably of the same species as in the positive sampling units. If other susceptible, unvaccinated animals are present, they could act as sentinels to provide additional serological evidence. The sentinels should be kept in close contact with the animals of the epidemiological unit under investigation for at least two incubation periods and should remain serologically negative if FMDV is not circulating.
Follow-up of field and laboratory findings
If transmission is demonstrated, an outbreak is declared.
The significance of small numbers of seropositive animals in the absence of current FMDV transmission is difficult to determine. Such findings may be an indication of past infection followed by recovery or by the development of a carrier state, in ruminants, or due to non-specific serological reactions. Antibodies to nonstructural proteins may be induced by repeated vaccination with vaccines that do not comply with the requirements for purity. However, the use of such vaccines is not permissible in countries or zones applying for an official status. In the absence of evidence of FMDV infection and transmission, such findings do not warrant the declaration of a new outbreak and the follow-up investigations may be considered complete.
However, if the number of seropositive animals is greater than the number of false positive results expected from the specificity of the diagnostic tests used, susceptible animals that have been in contact or otherwise epidemiologically associated with the reactor animals should be investigated further.
| Abbreviations and acronyms: | |
| ELISA | Enzyme-linked immunosorbent assay |
| VNT | Virus neutralisation test |
| NSP | Nonstructural protein(s) of foot and mouth disease virus (FMDV) |
| 3ABC | NSP antibody test |
| SP | Structural protein of foot and mouth disease virus |
Fig. 1. Schematic
representation of the minimum waiting periods and pathways for recovery
of FMD free status
after an outbreak in a free country or zone
where vaccination is not practised
Waiting periods are minima depending upon outcome of surveillance specified in respective articles. If there are multiple waiting periods because of different control measures, the longest applies.
Fig. 2. Schematic representation
of the minimum waiting periods and pathways for recovery of FMD
free status
after an outbreak in a free country or zone where
vaccination is practised
Waiting periods are minima depending upon outcome of surveillance specified in respective articles. If there are multiple waiting periods because of different control measures, the longest applies.
Fig. 3. Schematic representation
of laboratory tests for determining evidence of infection with FMDV
by
means of serological surveys
|
2016 ©OIE - Terrestrial Animal Health Code |